��Ŀ����
����Ŀ��ij�о���ѧϰС�����������װ����ȡ����֤SO2������
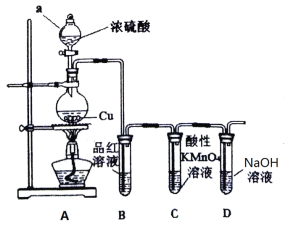
��ش�
��1��ͼ������a������Ϊ_____________��
��2��A�з�����Ӧ�Ļ�ѧ����ʽ��_____________________________________��
��3����C����Һ��ɫ������SO2����_________________�ԡ�
��4������NaOH��Һ��SO2��Ӧ�����ӷ���ʽ��__________________________________��
��5������֧װ��Ʒ����Һ���Թ��У��ֱ�ͨ��Cl2��SO2��������֧�Թ���Ʒ����Һ����ɫ���������ʵ��֤����֧�Թ���ͨ�����SO2��_________________��
��6����С��������ʵ�飬��ͬ�����½�Cl2��SO2�������尴���1��1��ϣ���ͨ��Ʒ����Һ�У��۲���Һ��������ɫ����������֪���������尴���1��1��ϣ�����ˮ��Ӧ���������ֳ������ᣬ���ʧȥƯ�����ã��÷�Ӧ�Ļ�ѧ����ʽ��________________________��
���𰸡���Һ©�� Cu + 2H2SO4 (Ũ) ![]() CuSO4 + SO2��+2H2O ��ԭ SO2+2OH= SO32 + H2O ����֧�Թ��е�Һ����ȣ��ָ���ɫ��ͨ���ΪSO2 Cl2+SO2+2H2O=H2SO4+2HCl
CuSO4 + SO2��+2H2O ��ԭ SO2+2OH= SO32 + H2O ����֧�Թ��е�Һ����ȣ��ָ���ɫ��ͨ���ΪSO2 Cl2+SO2+2H2O=H2SO4+2HCl
��������
A��Ũ������ͭ�ڼ��������·�Ӧ���ɶ��������ˮ������ͭ�������������Ư���ԣ��ܹ�ʹB��Ʒ����Һ��ɫ������������л�ԭ�ԣ��ܹ���C�и��������Һ��ɫ�����������ж�������Ķ������������D�������������գ��Դ˽����⡣
��1����װ��ͼ��֪����aΪ��Һ©�����ʴ�Ϊ����Һ©����
��2��Ũ�������ǿ�����ԣ����������¿���ͭ����������ԭ��Ӧ������ʽΪ![]() ��
��
��3��������ؾ���ǿ�����ԣ�C�и��������ɫ��˵������������л�ԭ�ԣ��ʴ�Ϊ����ԭ��
��4������NaOH��Һ��SO2��Ӧ�����������ƣ����ӷ���ʽ��SO2+2OH-=SO32-+H2O��
��5�����������Ư��Ч�����в��ȶ��ԣ����ȿɻָ���ԭ������ɫ��������������壬�ɽ���֧�Թ��е�Һ����ȣ��ָ���ɫ��ͨ���ΪSO2���ʴ�Ϊ������֧�Թ��е�Һ����ȣ��ָ���ɫ��ͨ���ΪSO2��
��6�����������������������ԭ��Ӧ������������ᣬ��Ӧ�ķ���ʽΪCl2+SO2+2H2O=H2SO4+2HCl��

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�����Ŀ�����������㷺����ҩ�ȼ�ϡ����ϵȹ�ҵ������ѧ��ѧʵ�����ﳣ����ͼװ�����Ʊ�����������![]() ���ּг���������ȥ
���ּг���������ȥ![]()
��֪��
�ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ�� | |
�Ҵ� | 0.79 | -114.5 | 78.4 | ��ˮ���� |
���� | 1.05 | 16.6 | 118.1 | ������ˮ���Ҵ� |
�������� | 0.90 | -83.6 | 77.2 | ����ˮ���������Ҵ� |
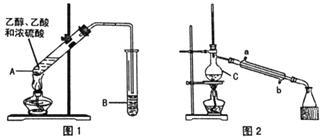
���Ʊ���Ʒ(ͼ1)
��A�м����������Ƭ��������ԭ�����μ���A�У��þƾ��ƻ������ȣ�һ��ʱ�����B�еõ�����������Ʒ��
��1��Ũ���ᡢ�Ҵ�������ļ���˳����___��A�з�����Ӧ�Ļ�ѧ����ʽ��___��
��2��A�����Ƭ��������___�������ܳ��˵����⣬�����е�������___��
��3��B��ʢװ��Һ����___���ռ���������������___��(��ϡ����¡�)��
��.�Ʊ���Ʒ(ͼ2)
��B�е�Һ���Һ��������������Ʒ����һϵ�г��Ӳ�����ת�Ƶ�C�У�����ͼ2װ�ý�һ���������õ�����������Ʒ��
��4��C��������___��
��5��ʵ������У���ȴˮ��___�ڽ���(����ĸ)���ռ���Ʒʱ�����Ƶ��¶�Ӧ��___���ҡ�
����Ŀ��������������NO��O2���ɣ���֪��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO(g)��O2(g)![]() 2NO2(g) ��H��n(NO)��n(O2)��ʱ��ı仯�����
2NO2(g) ��H��n(NO)��n(O2)��ʱ��ı仯�����
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.200 | 0.100 | 0.080 | 0.050 | 0.050 | 0.050 |
n(O2)/mol | 0.100 | 0.050 | 0.040 | 0.025 | 0.025 | 0.025 |
��1����֪��K800��>K1000������÷�Ӧ����H___0(��������������С����)����O2��ʾ0��2 s�ڸ÷�Ӧ��ƽ������Ϊ___��
��2����˵���÷�Ӧ�Ѵﵽƽ��״̬����___��
a��������������ɫ���ֲ��� b��2v��(NO)��v��(O2)
c��������ѹǿ���ֲ��� d�������������ܶȱ��ֲ���
��3��Ϊʹ�÷�Ӧ�������������NO��ת���ʣ���ƽ��������Ӧ�����ƶ���Ӧ��ȡ�Ĵ�ʩ��_____��
��4�������������£�����ͨ��2molNO��1molO2��ƽ�ⳣ��K��___��
��5�������������£�����ʼͨ�����0.2molNO2���壬�ﵽ��ѧƽ��ʱ��NO2��ת����Ϊ__��
��6��úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
��CH4(g)��4NO(g)![]() 2N2(g)��CO2(g)��2H2O ��H<0
2N2(g)��CO2(g)��2H2O ��H<0
��CH4(g)��2NO2(g)![]() N2(g)��CO2(g)��2H2O(g) ��H<0
N2(g)��CO2(g)��2H2O(g) ��H<0
���ڷ�Ӧ�ڣ������NO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��____��
a������ԭ�����ı���� b�������¶� c����СͶ�ϱ�[n(NO2)/n(CH4)] d������ѹǿ
����Ŀ������ԭCO2�ǽ������ЧӦ����Դ�������Ҫ�ֶ�֮һ���о���������Cu/ZnO���������£�CO2��H2�ɷ�������ƽ�з�Ӧ���ֱ�����CH3OH��CO����Ӧ���Ȼ�ѧ����ʽ���£�
CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1=-53.7kJ��mol-1 I
CH3OH(g)+H2O(g) ��H1=-53.7kJ��mol-1 I
CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2 II
CO(g)+H2O(g) ��H2 II
ijʵ���ҿ���CO2��H2��ʼͶ�ϱ�Ϊ1��2.2������ͬѹǿ�£�������ͬ��Ӧʱ��������ʵ�����ݣ�
T(K) | ���� | CO2ת���ʣ�%�� | �״�ѡ���ԣ�%�� |
543 | Cat.1 | 12.3 | 42.3 |
543 | Cat.2 | 10.9 | 72.7 |
553 | Cat.1 | 15.3 | 39.1 |
553 | Cat.2 | 12.0 | 71.6 |
����ע��Cat.1��Cu/ZnO���װ���Cat.2��Cu/ZnO����Ƭ���״�ѡ���ԣ�ת����CO2�����ɼ״��İٷֱ�
��֪����CO��H2�ı�ȼ���ȷֱ�Ϊ-283.0kJ��mol-1��-285.8kJ��mol-1
��H2O(l)=H2O(g) ��H3=44.0kJ��mol-1
��ش𣨲������¶ȶ���H��Ӱ�죩��
(1)��ӦI��ƽ�ⳣ������ʽK=___��
(2)���������CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��___��
A��ʹ�ô���Cat.1
B��ʹ�ô���Cat.2
C�����ͷ�Ӧ�¶�
D��Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ��
E������CO2��H2�ij�ʼͶ�ϱ�
(3)����ʵ�����ݱ���������ͬ�¶��²�ͬ�Ĵ�����CO2ת����CH3OH��ѡ������������Ӱ�죬��ԭ����___��
(4)��ͼ�зֱ���ӦI����������Cat.1����Cat.2��������¡���Ӧ���̡�������ʾ��ͼ___��