��Ŀ����
����Ŀ��ij��ѧС���Ա�����Ϊԭ����ȡ������������й����ʵķе����Է����������±���
���� | �״� | ������ | ��������� |
�е�/�� | 64.7 | 249 | 199.6 |
��Է������� | 32 | 122 | 136 |
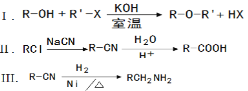
�����ϳɱ���������ֲ�Ʒ
����ƿ�м���12.2g�������20mL�״�(�ܶ�Լ0.79g/mL)����С�ļ���3mLŨ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ
��1�����Һ��ʱ������Ũ�����������_____________________��Ũ�����������_____________������Ӧ����ˮ�����м���ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ____________��
��2���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������____________��
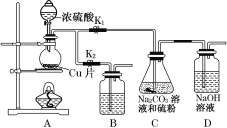
��3���ס��ҡ�����λͬѧ�ֱ��������ͼ1����ʵ������ȡ�����������װ��(�г������ͼ�������������ȥ)�������л�����ص㣬��ò���װ��________(��ס������ҡ���������)�� 
�����ֲ�Ʒ�ľ���
��4������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ�������ͼ2����ͼ���о��ƣ���� ������ͼ����ǡ���������������ƣ�����IΪ_______��������Ϊ _______��
��5��ͨ�����㣬����������IJ���Ϊ____________��
���𰸡���1��Ũ�����ܶȽϴ����뱽���ᡢ�״���Ϸų�����������������ˮ�� C6H5CO18OH+CH3OH![]() C6H5COOCH3+H218O
C6H5COOCH3+H218O
��2����ȴ��
��3����
��4����Һ ����
��5��65%
��������
�����������1��Ũ�����ܶȽϴ����뱽���ᡢ�״���Ϸų������������ʻ����ҺʱӦ������Ũ���Ũ������������Ӧ�����˴������ã�������ӦΪ���淴Ӧ��Ũ�������շ�Ӧ���ɵ�ˮ�����Դٽ��������ɣ�Ũ����������ˮ�������ã���������״���Ũ���������µ�������ӦΪ����������ȥ�ǻ����ݴ���ȥ�ǻ��е���ԭ�ӣ����߷�Ӧ���ɱ��������������ˮ��18Oԭ�����Ա����ᣬ��Ӧ�Ļ�ѧ����ʽΪ��C6H5CO18OH+CH3OH![]() C6H5COOCH3+H218O��
C6H5COOCH3+H218O��
��2���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ��������ȴ����룬��ֹҺ�屩�У�
��3����װ��ͼ��֪����ͼ��Բ����ƿ���������ܣ�����������ã���ͼ�ͱ�ͼ��û�У��������з�Ӧ��״��е�ͣ������ᡢ����������ķе�Զ���ڼ״��������ü�ͼ����ͼ���״��ض�������ӷ��������ںϳɷ�Ӧ������Ӧ�������������ټ״��Ļӷ�����߲��ʣ��ʴ�Ϊ�ң�
��4�����������������ˮ��������������������ܵ�Һ�壬ͨ�����÷�Һ������ɣ��״��ͱ����������ܽ⣬���߷е㲻ͬ������ͨ������������룻
��5��12.2g����������ʵ���Ϊ��![]() =0.1mol��20mL�״�(�ܶ�Լ0.79g/mL)�����ʵ���Ϊ��
=0.1mol��20mL�״�(�ܶ�Լ0.79g/mL)�����ʵ���Ϊ��![]() =0.49mol��0.1mol��
=0.49mol��0.1mol��
�����������ɱ�������������ʵ���Ϊ��0.1mol������Ϊ��136g/mol��0.1mol=13.6g����������IJ���Ϊ��![]() ��100%=65%��
��100%=65%��