��Ŀ����
����Ŀ���ҹ����зḻ��ͭ����Դ����ش������й�ͭ���仯��������⣺
(1)��д����̬Cuԭ�ӵļ۵����Ų�ʽ_________������е���ɫ��ͭ����ɫ����̬ͭԭ��������ʱ�۵��ӷ�����_________ ����Ϊ����̬��
(2)��ͭ����������ʹ�õĺϽ�֮һ����Ҫ��Zn��Cu��ɡ���һ������Il(Zn)______ Il(Cu)����������������С��������ԭ����___________��
(3)����ũҩ��֬��ͭ���еͲ������ص㣬��ͼ����֬��ͭ�Ľṹ��ʽ��

�����1����֬��ͭ�������ĸ���___________����*��̼ԭ�ӵ��ӻ���ʽΪ_____________��
(4)����ͭ���ڰ�ˮ�γ�![]() 4����ɫ��Һ��
4����ɫ��Һ��
�� ![]() �������ӵ����幹����_______��
�������ӵ����幹����_______��
����![]() ��
��![]() ֮���γɵĻ�ѧ����Ϊ_____���ṩ�µ��ӶԵijɼ�ԭ����_______________________��
֮���γɵĻ�ѧ����Ϊ_____���ṩ�µ��ӶԵijɼ�ԭ����_______________________��
�۰��ķе�_________________������������������������좣�
(5)��ͭ�Ͻ���Ա�ʾΪ![]() ��Ϊ�������������������ܶ�Ϊ8.5
��Ϊ�������������������ܶ�Ϊ8.5![]() �����ı߳�___________��ֻд����ʽ����������
�����ı߳�___________��ֻд����ʽ����������
���𰸡�3d104s1 ԾǨ ���� Zn��������Ų�Ϊȫ���ṹ����ʧȥ���� 6 sp3 �������� ��λ�� N ���� ![]()
��������
���ݺ�������Ų����ɷ�����𣻸��ݼ۲���ӶԻ������۷�������ӻ����͡��ռ乹�ͣ�������λ�����γɷ�����𣻸��ݾ����Ľṹ���ܶȽ�����ؼ��㡣
(1)Cuԭ��һ����29�����ӣ����ݺ�������Ų����ɼ����ع������������۵����Ų�ʽΪ3d104s1����ɫ��Ӧ���ǵ����ڲ�ͬ�Ĺ������ԾǨ����������
(2)Zn��Χ�����Ų�Ϊ3d104s2��Cu��Χ�����Ų�Ϊ3d104s1��Znԭ�Ӻ�������Ų�Ϊȫ���ṹ������ʧȥ���ӣ��ʵ�һ������п����ͭ���ʴ�Ϊ�����ڣ�Zn��������Ų�Ϊȫ���ṹ����ʧȥ���ӣ�
(3)������������˫������1��������1�����������л����к���3��2=6��˫��������1����֬��ͭ�������ĸ���Ϊ6������*��̼ԭ���γɵĶ��ǵ��������ӻ���ʽΪsp3��
(4)�� ![]() ��������Ϊ�����������ԭ��S�ļ۲���Ӷ���Ϊ4�������ӻ�����Ϊsp3��S��û�йµ��Ӷԣ�SO42-�����幹�����������壻
��������Ϊ�����������ԭ��S�ļ۲���Ӷ���Ϊ4�������ӻ�����Ϊsp3��S��û�йµ��Ӷԣ�SO42-�����幹�����������壻
����![]() ��
��![]() �ṩ�չ����
�ṩ�չ����![]() �ṩ�¶Ե��ӣ������γɵĻ�ѧ����Ϊ��λ�����ṩ�µ��ӶԵijɼ�ԭ����N��
�ṩ�¶Ե��ӣ������γɵĻ�ѧ����Ϊ��λ�����ṩ�µ��ӶԵijɼ�ԭ����N��
����Ϊ�������Ӽ�����γ�����������ķе����좣�
(5)�������Ϣ֪����ͭ�Ͻ���Ա�ʾΪCu3Zn��Ϊ�������������������к���3��Cu��1��Zn����������Ϊ��![]() g�����ı߳�Ϊ
g�����ı߳�Ϊ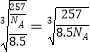 ��
��

 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�����Ŀ���±������������г��������ʣ������г������ǵģ���Ҫ���ɷ�
��� | �� | �� | �� | �� | �� | �� | �� |
���� | �ƾ� | ���� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
��Ҫ�ɷ� | CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
��1������Ա��Т�~�ߵ���Ҫ�ɷֽ��з��ࣨ���ţ���
���ڵ���ʵ���_______________ �����ڷǵ���ʵ���____________________ ��
��2�������ڵ�ˮ��Һ��߷�Ӧ�����ӷ���ʽ ________________________________��
��3����֪�ý�������ȡ�����ƣ����ж��ַ�����
��4Na + O2=2Na2O�� ��4Na + CO2 =2Na2O + C����2NaNO2 + 6Na =4Na2O + N2��
���������ַ����У���õ���_________ ��ԭ����___________��
��4��ijʵ��С��ͨ������ʵ��̽������������ˮ�ķ�Ӧ��

���û�ѧ����ʽ����ʹ��̪����ԭ�� _______________________________������ʵ�������Ʋ��ɫ��ȥ��ԭ����___________________________________��
�ڼ���MnO2��Ӧ�Ļ�ѧ����ʽΪ ___________________________________ ��
��5����ʵ����ģ������Ƽ�Ʊ�̼���ƣ�һ���¶��£���һ��������NaCl��Һ��ͨ�백���ﵽ���ͺ��ٲ���ͨ��CO2��һ��ʱ����ֳ��������˵õ�NaHCO3���塣�ù��̵Ļ�ѧ����ʽΪ��____________
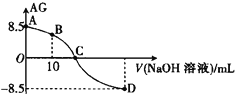
��6���ڻ�ѧ������ʦ��NaOH��Һͨ��CO2���������������������ͨ��ʵ��֤��CO2��NaOH�����˷�Ӧ��ij��ѧС��ͬѧ�������ĸɱ���������������Һ�У��������ֻ��������ⶨ��ҺpH�ı仯����ͼ��ʾ�����û�ѧ����ʽ�ش��������⣺
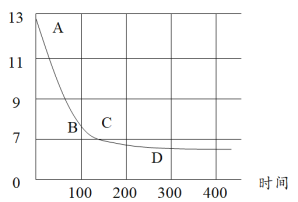
��BC�α仯ԭ������� ___________________________________________________________ ��
��CD�α仯ԭ������� ___________________________________________________________ ��