��Ŀ����
13����ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ��C��D����Է���������ȣ���EΪ��֧���Ļ����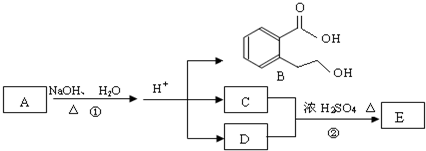
��ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ������E�ķ���ʽΪC5H10O2��
��2��������B���ܷ����ķ�Ӧ��e������ĸ��ţ���
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ d��������Ӧ e��ˮ�ⷴӦ f�� �û���Ӧ
��3����Ӧ�ڵĻ�ѧ����ʽCH3COOH+CH3CH2CH2OH $��_{��}^{Ũ����}$CH3COOCH2CH2CH3+H2O��
��4��C�����еĹ������������Ȼ���A�Ľṹ��ʽ��
 ��
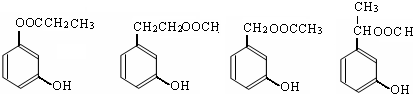
����4��ͬʱ������������������B��ͬ���칹�����Ŀ��4����
���м��ȡ�������ṹ
���������Ҳ��Ƿ������γɵ���
���� FeCl3��Һ������ɫ��Ӧ��
д��������������ͬ���칹��Ľṹ��ʽ
 ����2����
����2����
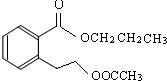
���� E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%����̼ԭ����ĿΪ$\frac{102��58.8%}{12}$=5��Hԭ����ĿΪ$\frac{102��9.8%}{1}$=10����ԭ����Ŀ=$\frac{102-12��5-10}{16}$=2����E�ķ���ʽΪC5H10O2��E��C��D��Ӧ���ɣ�C�ܺ�̼�����Ʒ�Ӧ����CΪ���ᣬDΪ�������߹���5��Cԭ�ӣ�����C��D����Է���������ȣ���CΪ���ᡢDΪ������E��֧����DӦΪCH3CH2CH2OH��EΪCH3COOCHCH2CH3��A�ڼ���������ˮ�⣬���ữ��B��C��D������A����B�����ᡢ��������������Ӧ�����ɵIJ����ṹ��ʽΪ�� ���ݴ˽��
���ݴ˽��
��� �⣺E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%����̼ԭ����ĿΪ$\frac{102��58.8%}{12}$=5��Hԭ����ĿΪ$\frac{102��9.8%}{1}$=10����ԭ����Ŀ=$\frac{102-12��5-10}{16}$=2����E�ķ���ʽΪC5H10O2��E��C��D��Ӧ���ɣ�C�ܺ�̼�����Ʒ�Ӧ����CΪ���ᣬDΪ�������߹���5��Cԭ�ӣ�����C��D����Է���������ȣ���CΪ���ᡢDΪ������E��֧����DӦΪCH3CH2CH2OH��EΪCH3COOCHCH2CH3��A�ڼ���������ˮ�⣬���ữ��B��C��D������A����B�����ᡢ��������������Ӧ�����ɵIJ����ṹ��ʽΪ�� ��
��
��1��ͨ�����Ϸ���֪��E�ķ���ʽΪ��C5H10O2���ʴ�Ϊ��C5H10O2��
��2��B�к��д��ǻ����Ȼ��ͱ��������д�������ͱ������ʣ��ܷ����ӳɷ�Ӧ��������Ӧ��ȡ����Ӧ��������Ӧ����ȥ��Ӧ���û���Ӧ����Ϊ������ԭ�ӻ����������Բ��ܷ���ˮ�ⷴӦ����ѡe��
��2����Ϊ����ͱ�����������Ӧ����Ӧ����ʽΪ��CH3COOH+CH3CH2CH2OH $��_{��}^{Ũ����}$CH3COOCH2CH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2CH2OH $��_{��}^{Ũ����}$CH3COOCH2CH2CH3+H2O��
��3��CΪ���ᣬ���й�����Ϊ�Ȼ���������������֪��AΪ ��
��
�ʴ�Ϊ���Ȼ��� ��
��
��5��B��ͬ���칹������������������м��ȡ�������ṹ�����������Ҳ��Ƿ������γɵ�����Ӧ��֬�����γɵ��������� FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ�������������ͬ���칹���У� ��
��
�ʴ�Ϊ��4��  ����2����
����2����
���� ���⿼���л�����ƶϣ��ؼ��Ǽ���ȷ��E�Ľṹ��ʽ����Ϸ�Ӧ������ת����ϵ���������ƻ����Ƶķ�ʽ�����ƶϣ���Ҫѧ���������չ�����������ת�����Ѷ��еȣ�

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�| A�� | ��ϩ�����ģ��Ϊ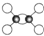 | |
| B�� | H2O2�ĵ���ʽΪ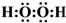 | |
| C�� | ����ĽṹʽΪCH4 | |
| D�� | ���������Ľṹ��ʽΪCH3COOCH3CH2 |
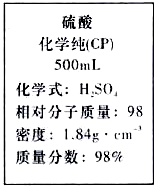
| A�� | ����������ʵ���Ũ��Ϊ9.2 mol•L-1 | |
| B�� | 1 mol �����뵽�����ĸ������У��ɲ���2 g H2 | |
| C�� | ���������������ˮ���������Һ�����ʵ���Ũ�ȴ���9.2 mol•L-1 | |
| D�� | ����200 mL 4.6 mol•L-1��������Һ��ȡ������50 mL |
����Һ���ν�������ʵ�飺��ͨ��һ������������CCl4�����ú�CCl4����Ϻ�ɫ���÷�Һ©��
��Һ�������Һ���ˮ��Һ�м������������ữ�����ᱵ��Һ��������ɫ�������۹��˺���Һ��Ϊ���ݣ�
��һ���м��������ữ����������Һ��������������������һ���м�������KSCN��Һ����Һ������Ϊ��
ɫ����������ʵ�飬�����Ʋ���ȷ���ǣ�������
| A�� | ԭ��Һһ�������� | |
| B�� | ԭ��Һ�п϶������ڵ�������Fe2+��Ba2+ | |
| C�� | ��ȷ��ԭ��Һ���Ƿ����Cl-��Na+��SO42- | |
| D�� | ԭ��Һ�п϶����ڵ�������Fe2+��SO42-��I- |
| A�� | c��CH3COO -����c��CH3COOH�� | B�� | c��CH3COO -����c��Na+����c��H+����c��OH -�� | ||
| C�� | c��Na+��=c��CH3COO -��=0.01mol•L-1 | D�� | c��CH3COOH��+c��CH3COO -��=0.02mol•L-1 |
| A�� | Cl-Cl����H-H���ļ���С������ΪClԭ�ӱ�Hԭ�ӵķǽ�����ǿ | |
| B�� | H2��g����Cl2��g����Ӧ����2 molHCl��g������Ӧ�ġ�H=183 kJ/mol | |
| C�� | H2��g����Cl2��g����Ӧ����2 molHCl��g������Ӧ�ġ�H=-183 kJ/mol | |
| D�� | H2��g����Cl2��g����Ӧ����1 molHCl��g������Ӧ�ġ�H=-183 kJ/mol |
| A�� | C2H4��C4H8 | B�� | C2H2��C3H8 | C�� | C3H4��C4H8 | D�� | C3H4��C3H8 |
| A�� | ����������̼ͨ��ƫ��������Һ�У�AlO2-+CO2+2H2O�TAl��OH��3��+HCO3- | |
| B�� | ����Һ�����������������ʵ����������ƻ�ϣ�NH4++HSO3-+2OH-�TSO32-+NH3��+2H2O | |
| C�� | ����ͨ������ռ���Һ�У�2Cl2+2OH-�T3Cl-+ClO-+H2O | |
| D�� | ̼����þ��Һ�м��������ʯ��ˮ��Mg2++2HCO3-+Ca2++2OH-�TCaCO3��+2H2O+MgCO3�� |