��Ŀ����
��Ӧ2C+O2===2CO�������仯����ͼ��ʾ������˵����ȷ����(����)
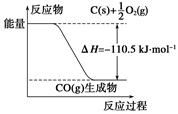
- A.12 g C(s)��һ����O2(g)��Ӧ����14 g CO(g)�ų�������Ϊ110.5 kJ
- B.�÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)+O2(g)===2CO(g)����H��-221 kJ
- C.2 mol C(s)������O2(g)��Ӧ����CO2(g)���ų�����������221 kJ
- D.�÷�Ӧ�ķ�Ӧ�ȵ���CO�����л�ѧ���γ�ʱ���ͷŵ���������O2�����л�ѧ������ʱ�����յ��������IJ�
C
�������������ͼʾ��Aѡ�����12 g C(s)��һ����O2(g)��Ӧ����28 g CO(g)�ų�������Ϊ110.5 kJ��Bѡ�����H�ĵ�λһ��ΪkJ��mol��1��kJ/mol�����÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)��O2(g)===2CO(g) ��H����221 kJ��mol��1��Cѡ����ȷ��2 mol C(s)��O2(g)��Ӧ����CO(g)�ų�������Ϊ221 kJ������CO(g)��O2(g)��Ӧ����CO2 (g)���ȣ����2 mol C(s)������O2 (g)��Ӧ����CO2(g)�ų�����������221 kJ��Dѡ����÷�Ӧ�ķ�Ӧ�ȵ���CO�����л�ѧ���γ�ʱ���ͷŵ���������C��O2�����л�ѧ������ʱ�����յ��������IJ��ѡC��
���㣺��ѧ��Ӧ�������仯
������ע���Ȼ�ѧ����ʽ����д�����ж��Լ���˹���ɵ�����,���ڳ���֪ʶ�㡣
�������������ͼʾ��Aѡ�����12 g C(s)��һ����O2(g)��Ӧ����28 g CO(g)�ų�������Ϊ110.5 kJ��Bѡ�����H�ĵ�λһ��ΪkJ��mol��1��kJ/mol�����÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)��O2(g)===2CO(g) ��H����221 kJ��mol��1��Cѡ����ȷ��2 mol C(s)��O2(g)��Ӧ����CO(g)�ų�������Ϊ221 kJ������CO(g)��O2(g)��Ӧ����CO2 (g)���ȣ����2 mol C(s)������O2 (g)��Ӧ����CO2(g)�ų�����������221 kJ��Dѡ����÷�Ӧ�ķ�Ӧ�ȵ���CO�����л�ѧ���γ�ʱ���ͷŵ���������C��O2�����л�ѧ������ʱ�����յ��������IJ��ѡC��
���㣺��ѧ��Ӧ�������仯
������ע���Ȼ�ѧ����ʽ����д�����ж��Լ���˹���ɵ�����,���ڳ���֪ʶ�㡣

��ϰ��ϵ�д�
 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ
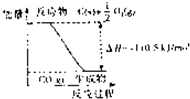 ��Ӧ2C+O2=2CO�������仯��ͼ��ʾ������˵����ȷ���ǣ�������
��Ӧ2C+O2=2CO�������仯��ͼ��ʾ������˵����ȷ���ǣ�������| A��12 g C��s����һ����O2��g����Ӧ����14 g CO��g�����ų�������Ϊ110.5 kJ | B��2 mol C��s��������02��g����Ӧ����CO2��g�����ų�����������221 kJ | C���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�2C��s��+O2��g��=2CO��g������H=-221kJ | D��̼��ȼ����Ϊ110.5kJ/mol |
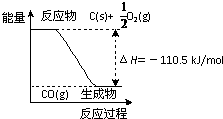 ��Ӧ2C+O2=2CO �������仯��ͼ��ʾ����11.2g KOH��ϡ��Һ��1L0.1mol/L��H2SO4��Һ��Ӧ�ų�11.46kJ������������˵����ȷ���ǣ�������
��Ӧ2C+O2=2CO �������仯��ͼ��ʾ����11.2g KOH��ϡ��Һ��1L0.1mol/L��H2SO4��Һ��Ӧ�ų�11.46kJ������������˵����ȷ���ǣ�������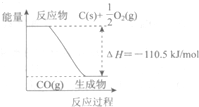 ��2010?������һģ����Ӧ2C+O2=2CO�������仯��ͼ��ʾ������˵����ȷ���ǣ�������
��2010?������һģ����Ӧ2C+O2=2CO�������仯��ͼ��ʾ������˵����ȷ���ǣ�������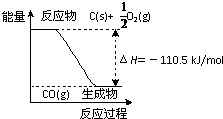 ��Ӧ2C+O2=2CO �������仯��ͼ��ʾ������˵����ȷ���ǣ�������
��Ӧ2C+O2=2CO �������仯��ͼ��ʾ������˵����ȷ���ǣ�������