��Ŀ����
����Ŀ��ij��п�����ú�ZnO���̳��ѳ����Ṥ�������е�SO2��ZnSO4��
��֪����ZnSO3��![]() H2O����ˮ��ZnSO4������ˮ��
H2O����ˮ��ZnSO4������ˮ��
��25��ʱ����Һ��S��+4�ۣ�����ֵ����ʵ���������pH�仯��������ͼ��
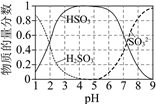
��O3Ϊǿ��������1 mol O3����ԭת��2 mol e��ͬʱ����1 mol O2��
(1)SO2��ɵĻ���������Ҫ��_____________________��
(2)ZnO��SO2ת��ΪZnSO3��![]() H2O�Ļ�ѧ����ʽ��_____________________________��
H2O�Ļ�ѧ����ʽ��_____________________________��
(3)��ZnSO3��![]() H2O����Һת��ΪZnSO4ʱ�����䱻O2���������ʺ���������ɹܵ�������ʵ����ģ��O3������������⡣��ʼʱ�����Ὣ5% ZnSO3����Һ���� pH��3.5����һ���Ľ�������ͨ��O3���о�O3����ZnSO3��
H2O����Һת��ΪZnSO4ʱ�����䱻O2���������ʺ���������ɹܵ�������ʵ����ģ��O3������������⡣��ʼʱ�����Ὣ5% ZnSO3����Һ���� pH��3.5����һ���Ľ�������ͨ��O3���о�O3����ZnSO3��![]() H2O��������ҺpH��ʱ��仯���������£�
H2O��������ҺpH��ʱ��仯���������£�

��pH��3.5����Һ�к�S��+4�ۣ���������Ҫ��____________________��
��һ��ʱ�����Һ��pH��С���˹�������Ҫ��Ӧ�����ӷ���ʽ��________________��
�۽���O3�����ɷ�ֹ�ܵ�������ԭ��___________________��
���𰸡����� 2ZnO+2SO2+5H2O=2ZnSO3��![]() H2O HSO3 HSO3+O3=SO42+O2+H+��2HSO3+O3=SO42+O2+H2SO3�� O3��ZnSO3��
H2O HSO3 HSO3+O3=SO42+O2+H+��2HSO3+O3=SO42+O2+H2SO3�� O3��ZnSO3��![]() H2O����Ϊ���ܵ�ZnSO4�Ļ�ѧ��Ӧ���ʿ죬��λʱ���ڹ���������٣���ֹ�ܵ�����
H2O����Ϊ���ܵ�ZnSO4�Ļ�ѧ��Ӧ���ʿ죬��λʱ���ڹ���������٣���ֹ�ܵ�����
��������
(1)SO2����������Ҫ���壻
(2)ZnO��SO2�Լ�ˮ��Ӧ����ZnSO3��![]() H2O��
H2O��
(3)�ٸ���25��ʱ����Һ��S(+4��)����ֵ����ʵ���������pH�仯���߷����ж�pH��3.5����Һ�к�S(+4��)�����ӣ�
��һ��ʱ�������ͨ��O3����HSO3����ΪSO42��������ǿ����ʣ���Һ��pH��С��
��O3�����ܵ�ZnSO3��![]() H2O����Ϊ���ܵ�ZnSO4����Ӧ���ʼӿ졣
H2O����Ϊ���ܵ�ZnSO4����Ӧ���ʼӿ졣
(1)SO2��ɵĻ���������Ҫ�����ꣻ
(2) ZnO��SO2�Լ�ˮ��Ӧ����ZnSO3��![]() H2O����ѧ����ʽ��2ZnO+2SO2+5H2O=2ZnSO3��
H2O����ѧ����ʽ��2ZnO+2SO2+5H2O=2ZnSO3��![]() H2O��
H2O��
(3)������25��ʱ����Һ��S(+4��)����ֵ����ʵ���������pH�仯���ߣ�pH��3.5����Һ��HSO3Ũ��ԶԶ����H2SO3��pH��3.5����Һ�к�S(+4��)��������Ҫ��HSO3��
��һ��ʱ�������ͨ��O3����HSO3����ΪSO42��������ǿ����ʣ�HSO3�������ӱ��ͷţ���Һ��pH��С�����ӷ���ʽ�ǣ�HSO3+O3=SO42+O2+H+(��2HSO3+O3=SO42+O2+H2SO3)��
��O3��ZnSO3��![]() H2O����Ϊ���ܵ�ZnSO4�Ļ�ѧ��Ӧ���ʿ죬��λʱ���ڹ���������٣���ֹ�ܵ�������
H2O����Ϊ���ܵ�ZnSO4�Ļ�ѧ��Ӧ���ʿ죬��λʱ���ڹ���������٣���ֹ�ܵ�������

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�����Ŀ����ͼ��ʾ����֧�缫��Ϊ���缫����ѡ�������������Һ���±���
�� | A | B | C | D |
�ײ� | NaOH��Һ | AgNO3��Һ | H2SO4��Һ | NaCl��Һ |
�Ҳ� | CuSO4��Һ | CuCl2��Һ | AgNO3��Һ | AgNO3��Һ |
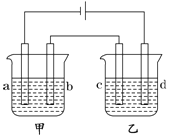
Ҫ����������ǣ��ٹ���һ��ʱ��ײ۵��ҺpH���������Ҳ۵��ҺpH�½�����b��c�����ŵ����ӵ����ʵ�����ȡ���
(1)Ӧѡ�õĵ��Һ��________�顣
(2)�ײ۵ĵ�ⷽ��ʽΪ��_____________���Ҳ۵ĵ�ⷽ��ʽΪ��_______________��