��Ŀ����
7����1����ѹǿΪO��1MPa�����£��ݻ�ΪV L���ܱ������У�amol CO��2amol H2�ڴ��������·�Ӧ���ɼ״�����Ӧ�Ļ�ѧ����ʽΪCO��g��+2H2��g��?CH3OH��g����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ3��ʾ����
��P1��P2���������������=������
���������������������£�������������a molCO��2amol H2���ﵽ��ƽ��ʱ��CO��ת���������������С�����䡱����ͬ����ƽ�ⳣ�����䣮
����P1�¡�100��ʱ��CH3OH��g��?CO��g��ʮ2H2��g����ƽ�ⳣ��Ϊ$\frac{{a}^{2}}{{v}^{2}}$���ú�a��V�Ĵ���ʽ
��ʾ����
��2���ٳ����£�����2mol NH3��g����l mol CO2��g��ͨ��1Lˮ�У��ɵ�pH=10����Һ�������Һ��Ũ��������������CO32-��
�ڳ����£���O��01mol•L-1 NaOH��Һ��0.01mol•L-1��NH4��2SO4��Һ�������ϣ�������ҺpHΪ10����ô�û��Һ��c��Na+��+c��NH4+��=10-2+10-4-10-10mol•L-1����ȷ����ʽ�����ܹ��㣩��
���� ��1������ͬ�¶��£�ͬһ�����У�����ѹǿ��ƽ��������Ӧ�����ƶ�����CO��ת��������
���¶��ݻ����䣬����ܱ�����������a mol CO�� 2a mol H2����ЧΪ��ʼ����2a mol CO�� 4a mol H2���������1����ƽ�������ѹǿ����ѹ���ָ���ԭ�����������ѹǿƽ���������С�ķ����ƶ���ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣻
�۸��¶��£�ƽ��ʱn��CO��=amol����1-0.5��=0.5amol��n��CH3OH��=c��CO�����μӷ�Ӧ��=amol��0.5=0.5amol��n��H2��=2amol-2��amol��0.5=amol����c��CO��=$\frac{0.5a}{V}$mol/L��c��CH3OH��=$\frac{0.5a}{V}$mol/L��c��H2��=$\frac{a}{V}$mol/L����ѧƽ�ⳣ��K=$\frac{c��C{H}_{3}OH��}{c{��CO����c}^{2}��{H}_{2}��}$��
��2���ٽ�2mol NH3��g����l mol CO2��g��ͨ��1Lˮ�У�ǡ������̼��泥��ݴ˷������ɣ�
�����ݵ�����ԭ���г�ʽ�ӣ��������ݼ��㼴�ɣ�
��� �⣺��1������ͬ�¶��£�ͬһ�����У�����ѹǿ��ƽ��������Ӧ�����ƶ�����CO��ת����������ͼ��֪��p1С��p2���ʴ�Ϊ��С�ڣ�
���¶��ݻ����䣬����ܱ�����������a mol CO�� 2a mol H2����ЧΪ��ʼ����2a mol CO�� 4a mol H2���������1����ƽ�������ѹǿ����ѹ���ָ���ԭ�����������ѹǿƽ���������С�ķ����ƶ����÷�ӦΪ���������С�ķ�Ӧ����������Ӧ�ƶ���COת��������ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣮
�ʴ�Ϊ�������䣻
�۸��¶��£�ƽ��ʱn��CO��=amol����1-0.5��=0.5amol��n��CH3OH��=c��CO�����μӷ�Ӧ��=amol��0.5=0.5amol��n��H2��=2amol-2��amol��0.5=amol����c��CO��=$\frac{0.5a}{V}$mol/L��c��CH3OH��=$\frac{0.5a}{V}$mol/L��c��H2��=$\frac{a}{V}$mol/L����ѧƽ�ⳣ��K=$\frac{c{��CO����c}^{2}��{H}_{2}��}{c��C{H}_{3}OH��}$=$\frac{\frac{0.5a}{V}����\frac{a}{V}��^{2}}{\frac{0.5a}{V}}$=$��\frac{a}{V}��^{2}$���ʴ�Ϊ����$\frac{a}{V}$��2��
��2���ٽ�2mol NH3��g����l mol CO2��g��ͨ��1Lˮ�У�ǡ������̼��泥���ѧ��Ӧ����ʽΪ��2NH3+CO2+H2O=��NH4��2CO3������Һ�д���Ũ��������������̼������ʴ�Ϊ��CO32-��
����������Һ�����ΪV����Ϻ���Һ�е��������ǣ�Na+��NH4+��H+���������ǣ�OH-��SO42-�����ݵ�����ԭ���У�c��Na+��+c��NH4+��+c��H+��=2c��SO42-��+c��OH-������c��Na+��+c��NH4+��=2c��SO42-��+c��OH-��-c��H+��=2��$\frac{0.01mol/L��1L}{1L+1L}$+$\frac{1{0}^{-14}}{1{0}^{-10}}$-10-10=0.01+10-4-10-10=10-2+10-4-10-10���ʴ�Ϊ��10-2+10-4-10-10��
���� ������Ҫ������ǿ��淴Ӧת�������¶Ⱥ�ѹǿ�仯�����ߣ�Ҫ��ѧ���߱�����ͼ����������Ϣ��ȡ������������������漰ƽ�ⳣ���ļ��㡢��Һ������Ũ�ȴ�С�Ƚ��Լ�������ԭ���Ӧ�ã���һ���Ѷȣ�

| A�� | V1��V2��11��1 | B�� | V1��V2��9��1 | C�� | V1��V2��11��1 | D�� | V1��V2��1��9 |
| A�� | ˮ���������H+Ũ��Ϊ1��10-12mol•L-1����Һ��NH4+��Na+��C1-��HCO3- | |
| B�� | ���д���Fe3+����Һ��SCN-��I-��K+��Br- | |
| C�� | ��ʹpH��ֽ����ɫ����Һ��Na+��S2-��CO32-��AlO2- | |
| D�� | ����������ɫ��Һ��ClO-��MnO4-��A13+��SO42- |
| A�� | ������ʣ�࣬��Һ��dz��ɫ��Cl-�������ֲ��� | |
| B�� | ����Һ�е�����ɫKSCN��Һ����Һ���ɫ | |
| C�� | Fe2+��Fe3+���ʵ���֮��Ϊ5��1 | |
| D�� | ��������ͻ�ԭ��������ʵ���֮��Ϊ2��5 |
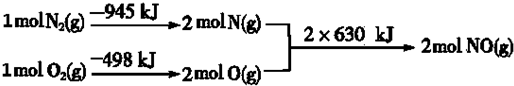
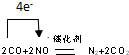 ��
��