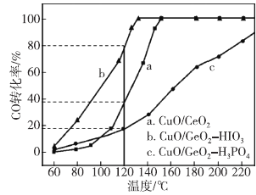
ЬтФПФкШн
ЁОЬтФПЁПСЌЖўбЧСђЫсФЦ(Na2S2O4)ЫзГЦБЃЯеЗлЃЌГЃгУгкЗФжЏЙЄвЕЁЂЪГЦЗЦЏАзЕШСьгђЁЃФГжжNa2S2O4ЕФЩњВњЙЄвеСїГЬШчЭМЫљЪОЃКШєдкЪЕбщЪвФЃФтИУЙЄвеСїГЬЃЌЯТСаЫЕЗЈДэЮѓЕФЪЧ
A.НЋаПЗлЭЖШыЫЎжааЮГЩаќИЁвКжївЊЪЧЮЊСЫМгПьЗДгІЫйТЪ
B.ЯђNa2S2O4ШмвКжаМгNaClШмвКЛђЙЬЬхЖдNa2S2O4ЕФВњТЪЮоУїЯдгАЯь
C.ЯДЕгNa2S2O4ЁЄ2H2OЪБгУБљЫЎаЇЙћКУгкГЃЮТеєСѓЫЎ
D.ИУСїГЬжаЩцМАЛЏКЯЗДгІЁЂЗжНтЗДгІЁЂИДЗжНтЗДгІЃЌВЛЩцМАбѕЛЏЛЙдЗДгІ
ЁОД№АИЁПBD
ЁОНтЮіЁП
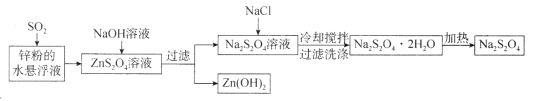
гЩСїГЬЭМПЩжЊЃЌаПЗлгыЖўбѕЛЏСђЗДгІЩњГЩZnS2O4ЃЌЯђZnS2O4ШмвКжаМгШыЧтбѕЛЏФЦШмвКЃЌСНепЗДгІЩњГЩЧтбѕЛЏаПГСЕэКЭNa2S2O4ЃЌЙ§ТЫКѓЃЌЯђNa2S2O4ШмвКжаМгШыNaClЙЬЬхНЕЕЭNa2S2O4ШмНтЖШЃЌЮіГіNa2S2O4ЁЄ2H2OОЇЬхЃЌЙ§ТЫКѓЃЌМгШШNa2S2O4ЁЄ2H2OОЇЬхЭбШЅНсОЇЫЎЩњГЩNa2S2O4ЁЃ
A.НЋаПЗлЭЖШыЫЎжааЮГЩаќИЁвКПЩдіДѓгыЖўбѕЛЏСђЕФНгДЅУцЛ§ЃЌМгПьЗДгІЫйТЪЃЌЙЪAе§ШЗЃЛ
B.Мг![]() ЙЬЬхКЭРфШДНСАшЖМгаРћгкШмНтЦНКтФцЯђвЦЖЏЃЌПЩЮіГіИќЖрЕФ
ЙЬЬхКЭРфШДНСАшЖМгаРћгкШмНтЦНКтФцЯђвЦЖЏЃЌПЩЮіГіИќЖрЕФ![]() ЃЌШєЪЙгУ
ЃЌШєЪЙгУ![]() ШмвКЃЌЛсНЕЕЭФЦРызгХЈЖШЪЙЮіГіЕФ
ШмвКЃЌЛсНЕЕЭФЦРызгХЈЖШЪЙЮіГіЕФ![]() МѕЩйЃЌ
МѕЩйЃЌ![]() ВњТЪНЕЕЭЃЌЙЪBДэЮѓЃЛ
ВњТЪНЕЕЭЃЌЙЪBДэЮѓЃЛ
C.гУБљЫЎЯДЕгПЩМѕЩй![]() ЕФЫ№ЪЇЃЌЙЪCе§ШЗЃЛ
ЕФЫ№ЪЇЃЌЙЪCе§ШЗЃЛ
D.аПЗлгыЖўбѕЛЏСђЕФЗДгІЪЧЛЏКЯЗДгІЃЌЗДгІжадЊЫигаЛЏКЯМлБфЛЏЃЌвВЪЧбѕЛЏЛЙдЗДгІЃЌ![]() ШмвКгы
ШмвКгы![]() ШмвКЕФЗДгІЪЧИДЗжНтЗДгІЃЌМгШШ
ШмвКЕФЗДгІЪЧИДЗжНтЗДгІЃЌМгШШ![]() ЕФЗДгІЪЧЗжНтЗДгІЃЌЙЪDДэЮѓЃЛ
ЕФЗДгІЪЧЗжНтЗДгІЃЌЙЪDДэЮѓЃЛ
ЙЪбЁBDЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁПдквЛЬхЛ§ПЩБфЕФУмБеШнЦїжаЃЌМгШывЛЖЈСПЕФXЁЂYЃЌЗЂЩњЗДгІЃКmX(g)![]() nY(g)ЁЁІЄHЃНQ kJ/molЁЃЗДгІДяЕНЦНКтЪБЃЌYЕФЮяжЪЕФСПХЈЖШгыЮТЖШЁЂЦјЬхЬхЛ§ЕФЙиЯЕШчЯТБэЫљЪОЃК
nY(g)ЁЁІЄHЃНQ kJ/molЁЃЗДгІДяЕНЦНКтЪБЃЌYЕФЮяжЪЕФСПХЈЖШгыЮТЖШЁЂЦјЬхЬхЛ§ЕФЙиЯЕШчЯТБэЫљЪОЃК
1L | 2L | 4L | |
100Ёц | 1.00mol/L | 0.75mol/L | 0.53mol/L |
200Ёц | 1.20mol/L | 0.90mol/L | 0.63mol/L |
300Ёц | 1.30mol/L | 1.00mol/L | 0.70mol/L |
ЯТСаЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
A.ЮТЖШВЛБфЃЌбЙЧПдіДѓЃЌYЕФжЪСПЗжЪ§МѕЩй
B.ЬхЛ§ВЛБфЃЌЮТЖШЩ§ИпЃЌЦНКтЯђФцЗДгІЗНЯђвЦЖЏ
C.mЃОn
D.QЃМ0