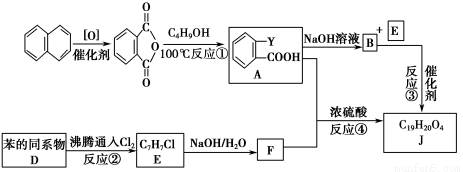
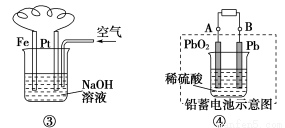
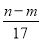̀âÄ¿ÄÚÈƯ
Èơµç½âÖʵĵçÀëƽºâ¡¢ÑÎÀàµÄË®½âƽºâºÍÄÑÈÜÎïµÄÈܽâƽºâ¾ùÊôÓÚ»¯Ñ§Æ½ºâ¡£
̉ÑÖªH2AÔÚË®ÖĐ´æÔÚ̉ÔÏÂƽºâ£ºH2A=H£«£«HA££¬HA£ H£«£«A2£¡£
H£«£«A2£¡£
(1)³£ÎÂÏÂNaHAÈÜ̉ºµÄpH________(̀îĐ̣ºÅ£¬ÏÂͬ)£¬Ộ̉ÊÇ_____________¡£
A£®´óÓÚ7¡¡¡¡¡¡¡¡¡¡¡¡B£®Đ¡ÓÚ7 C£®µÈÓÚ7 D£®Î̃·¨È·¶¨
(2)ijζÈÏ£¬ÈôỊ̈0.1 mol¡¤L£1µÄNaHAÈÜ̉ºÖĐÖđµÎµÎ¼Ó0.1 mol¡¤L£1 KOHÈÜ̉ºÖÁÈÜ̉º³ÊÖĐĐÔ(ºöÂÔ»́ºÏºóÈÜ̉ºµÄ̀å»ư±ä»¯)¡£´Ëʱ¸Ă»́ºÏÈÜ̉ºÖеÄÏÂÁĐ¹Øϵ̉»¶¨ƠưÈ·µÄÊÇ________¡£
A£®c(H£«)¡¤c(OH£)£½1.0¡Á10£14
B£®c(Na£«)£«c(K£«)£½c(HA£)£«2c(A2£)
C£®c(Na£«)>c(K£«)
D£®c(Na£«)£«c(K£«)£½0.05 mol¡¤L£1
(3)̉ÑÖª³£ÎÂÏÂH2AµÄ¸ÆÑÎ(CaA)µÄ±¥ºÍÈÜ̉ºÖĐ´æÔÚ̉ÔÏÂƽºâ£ºCaA(s)??Ca2£«(aq)£«A2£(aq)¡¡¦¤H>0¡£ÈổªÊ¹¸ĂÈÜ̉ºÖĐCa2£«Å¨¶È±äĐ¡£¬¿É²ÉÈ¡µÄ´ëÊ©ÓĐ________¡£
A£®Éư¸ßζȡ¡¡¡¡¡¡¡¡¡¡¡B£®½µµÍζÈ
C£®¼ÓÈëNH4Cl¾§̀å D£®¼ÓÈëNa2A¹̀̀å
¡¡(1)B¡¡HA£Ö»µçÀ벻ˮ½â£¬ÏÔËáĐÔ
(2)BC¡¡(3)BD
¡¾½âÎö¡¿¡¡H2AÔÚË®ÖеÄ̉»¼¶µçÀë½øĐĐÍêÈ«£¬ỘHA£²»Ë®½âÖ»µçÀ룬¹ÊNaHAÈÜ̉º³ÊËáĐÔ¡£HA£ÔÚË®ÖĐ²¿·ÖµçÀ룬0.1 mol¡¤L£1µÄNaHAÈÜ̉ºÖĐc(H£«)Đ¡ÓÚ0.1 mol¡¤L£1£¬¼ÓÈë0.1 mol¡¤L£1 KOHÈÜ̉ºÖÁÈÜ̉º³ÊÖĐĐÔʱÏûºÄµÄKOHÈÜ̉º̀å»ưĐ¡ÓÚNaHAÈÜ̉º̀å»ư£¬Ộ»́ºÏÈÜ̉ºÖĐc(Na£«)>c(K£«)£»ÓɵçºÉÊغăÖª£¬c(Na£«)£«c(K£«)£«c(H£«)£½c(HA£)£«2c(A2£)£«c(OH£)£¬Ç̉c(H£«)£½c(OH£)£¬Ộc(Na£«)£«c(K£«)£½c(HA£)£«2c(A2£)£»Ë®µÄÀë×Ó»ưÓëζÈÓĐ¹Ø£»½µÎ¡¢Ôö´óc(A2£)¶¼ÄÜʹƽºâCaA(s)??Ca2£«(aq)£«A2£(aq)×ó̉Æ¡£