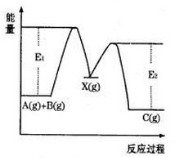
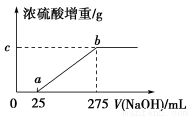
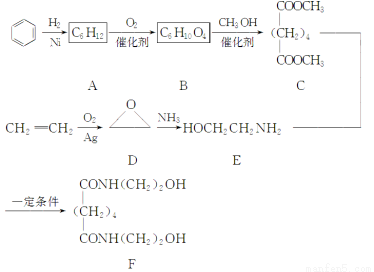
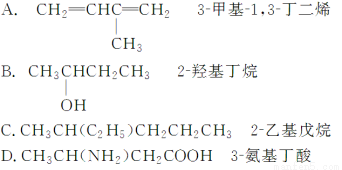
��Ŀ����
��ȡm gþ���Ͻ���һ��Ũ�ȵ�ϡ������ǡ����ȫ�ܽ�(����Ļ�ԭ����ֻ��NO)����Ӧ��Ļ����Һ�еμ�b mol/L NaOH��Һ�����μӵ�V mLʱ���õ���������ǡ��Ϊ���ֵn g���������йظ�ʵ���˵������ȷ����(����)
��������OH��������Ϊ(n��m)g
��ǡ���ܽ����Һ�е�NO3�������ʵ���Ϊ  mol
mol
����Ӧ������ת�Ƶĵ�����Ϊ 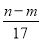 mol
mol
����״��������NO�����Ϊ  L
L
����Ͻ�Ӧ����������ʵ���Ϊ( ��
�� )mol
)mol
A��5�������B��4�� C��3�� D��2��
��A
���������������漰�ķ�Ӧ��Al��4HNO3(ϡ)=Al(NO3)3��NO����2H2O��3Mg��8HNO3(ϡ)=3Mg(NO3)2��2NO����4H2O��Al(NO3)3��3NaOH=Al(OH)3����3NaNO3��Mg(NO3)2��2NaOH=Mg(OH)2����2NaNO3�����Ͻ�ǡ���ܽ�ʱ����Һ�е�NO3����Na�������ʵ�����ȣ�n(NO3��)��n(NaOH)�� mol��������ȷ�������������ʱ�����ɵ�n g����Ϊ����������������þ�����������غ㶨�ɣ�����þ����Ԫ�ص���������m g�����Գ�����������������Ϊ(n��m)g����Ӧ������ת�Ƶĵ�����n(e��)��n(OH��)��
mol��������ȷ�������������ʱ�����ɵ�n g����Ϊ����������������þ�����������غ㶨�ɣ�����þ����Ԫ�ص���������m g�����Գ�����������������Ϊ(n��m)g����Ӧ������ת�Ƶĵ�����n(e��)��n(OH��)��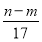 mol������������ȷ�����ݵ��ӵ�ʧ�غ�֪����״����V(NO)��
mol������������ȷ�����ݵ��ӵ�ʧ�غ�֪����״����V(NO)�� L��������ȷ���μӷ�Ӧ���������������ã����������õ�����(����������)�����ʵ������������Ƶ����ʵ�������
L��������ȷ���μӷ�Ӧ���������������ã����������õ�����(����������)�����ʵ������������Ƶ����ʵ������� mol��������������������ʵ�������NO�����ʵ�������
mol��������������������ʵ�������NO�����ʵ�������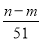 mol�����ԣ���Ͻ�Ӧ����������ʵ���Ϊ(
mol�����ԣ���Ͻ�Ӧ����������ʵ���Ϊ(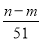 ��
�� )mol��������ȷ��
)mol��������ȷ��

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�