��Ŀ����
11���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺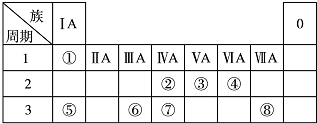
��1���ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ���ǵ������ڵڢ�A�壮
��2���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������һ�ֻ�����ĵ���ʽ
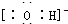 ��
����3���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬�������ʲ������÷�Ӧ�������ǣ�����ţ�bc��
a��MnO2 ��b��HI c��Na2SO3 d��FeCl3
��4��W��������ڵ�ͬ����Ԫ�أ����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3+3H3PO3�T3H3PO4+H2W�� |
| 1 | ||
| 2 |
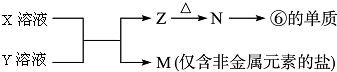
X��Һ��Y��Һ��Ӧ�����ӷ���ʽAl3++3NH3+3H2O�TAl��OH��3��+3NH4+��M�������ӵļ�������ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
���� ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
��1���ؿ��к������ڵڶ�λ��Ԫ��ΪSi��
��2����H��O��Na��Cl�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ�������NaOH��NaClO��Na2O2�ȣ�
��3����������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������ΪH2O2����MnO2��FeCl3�������·ֽ⣬��HI��Na2SO3���л�ԭ�ԣ���H2O2����������ԭ��Ӧ��
��4��W��������ڵ�ͬ����Ԫ�أ�������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ�
��5��M�ǽ����ǽ�����������һ������Σ���ΪAlԪ�أ������ƶ�N������������Z��������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪���÷�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ���ݴ˽��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪCl��
��1���ؿ��к������ڵڶ�λ��Ԫ��ΪSi���������ڱ��е������ڵڢ�A�壬
�ʴ�Ϊ���������ڵڢ�A�壻
��2����H��O��Na��Cl�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ�������NaOH��NaClO��Na2O2�ȣ�����NaOH�ĵ���ʽΪ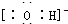 ��
��
�ʴ�Ϊ��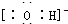 ��
��
��3����������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������ΪH2O2����MnO2��FeCl3�������·ֽ⣬��HI��Na2SO3���л�ԭ�ԣ���H2O2����������ԭ��Ӧ��������������
�ʴ�Ϊ��bc��
��4��W��������ڵ�ͬ����Ԫ�أ�������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ����Ա�ǿ��������������H2SO3+Br2+2H2O=H2SO3+2HBr����NaOH�����кͷ�ӦH2SO3+2NaOH=Na2SO3+2H2O��
�ʴ�Ϊ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3+3H3PO3�T3H3PO4+H2W�� |
| 1 | ��ԭ�� | H2SO3+Br2+2H2O�TH2SO3+2HBr |
| 2 | ���� | H2SO3+2NaOH�TNa2SO3+2H2O |
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ��Al3++3NH3+3H2O�TAl��OH��3��+3NH4+��
M�������ӵļ�������Ϊ��ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
�ʴ�Ϊ��Al3++3NH3+3H2O�TAl��OH��3��+3NH4+��ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
���� ���⿼��Ԫ�����ڱ��������ƶϡ����û�ѧ���Ԫ�ػ��������ʵȣ��Ѷ��еȣ�ע�����ճ������Ӽ���ȣ�

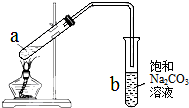
| A�� | ��a�Թ����ȼ���Ũ���ᣬȻ���ҡ���Թܱ����������Ҵ����ټ�������� | |
| B�� | �Թ�b�е������¶˹ܿڲ�������Һ���Ŀ���Ƿ�ֹʵ������в����������� | |
| C�� | ʵ��ʱ�����Թ�a��С�ľ��ȼ��ȵ�ԭ���DZ���Һ����ҷ��ڣ������Ҵ�������Ļӷ�����ֹ�¶ȹ���ʱ����̿�� | |
| D�� | �Թ�b�б���Na2CO3��Һ����������������������������������������� |
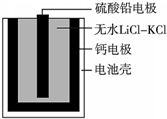 �ȼ����ؿ���������������Ĺ�����Դ��һ���ȼ����صĻ����ṹ��ͼ��ʾ��������Ϊ����ʵ���ˮLiCl-KCl������������ں�ؼ���˲��������ܣ��õ���ܷ�ӦΪ��PbSO4+2LiCl+Ca�TCaCl2+Li2SO4+Pb�������й�˵����ȷ���ǣ�������
�ȼ����ؿ���������������Ĺ�����Դ��һ���ȼ����صĻ����ṹ��ͼ��ʾ��������Ϊ����ʵ���ˮLiCl-KCl������������ں�ؼ���˲��������ܣ��õ���ܷ�ӦΪ��PbSO4+2LiCl+Ca�TCaCl2+Li2SO4+Pb�������й�˵����ȷ���ǣ�������| A�� | ������Ӧʽ��Ca+2Cl--2e-�TCaCl2 | |
| B�� | �ŵ�����У�Li+���ƶ� | |
| C�� | ÿת��0.1 mol���ӣ�����������20.7 g Pb | |
| D�� | ����ʱ��������������ϵ�����������ƣ�ָ�벻ƫת |

��������������ǣ�������
| A�� | ���裨1�������в����ı��ӿ���FeCl3��Һ���� | |
| B�� | ���裨2�������в�����ϩ����������ˮ���� | |
| C�� | ��������Ͳ�����������NaOH��Һ������Ӧ | |
| D�� | ���ӺͲ���������������KMnO4��Һ������Ӧ |
�ֱ���ʢ��0.5g Na2CO3���塢0.5gNaHCO3������ձ��м���10mLˮ��20�棩�����裬�����¶�ΪT1��
���ú��º�����¶�ΪT2��
�ֱ����10mL �ܶ�ԼΪ1.1g/mL 20%�����ᣨ20�棩�����裬�����¶�T3��
�õ��±������ݣ�
| �¶� �Լ� | T1/�� | T2/�� | T3/�� |
| NaCO3 | 23.3 | 20.0 | 23.7 |
| NaHCO3 | 18.5 | 20.0 | 20.8 |
��1��NaHCO3����ˮ�Լ��ԣ���ԭ����HCO3-+H2O?H2CO3+OH-�������ӷ���ʽ��ʾ����
��2�����������ĸ����жϣ��������Na2CO3��NaHCO3�����ܷ�ȫ���ܽ��ǣ���ǡ�����
��3��������1�����ݵó���Na2CO3��������ˮ���ȣ�NaHCO3��������ˮ���ȣ�����ȡ������ȡ�����
��4����ͬѧ�����������ݵó���Na2CO3��NaHCO3�����ᷴӦ���Ƿ��ȷ�Ӧ��
��ͬѧ��ΪӦ������һ��ʵ�飬����������ʵ�飺�� ʢ ��10mLˮ��20�棩���ձ��м���10mL�ܶ�ԼΪ1.1g/mL20%�����ᣨ20�棩�����裬�����¶�Ϊ22.2�森
��5���������̽��������˵����ȷ����AC��
A��NaHCO3������ķ�Ӧ�����ȷ�Ӧ
B��������ϡ�������Na2CO3��NaHCO3����
C��Na2CO3��NaHCO3������ϡ���ᷴӦ�������仯�������ʵ��ܽ�������й�
��6����ͬѧΪ�ⶨһ��NaHCO3��Na2CO3��Ϲ�����NaHCO3�Ĵ��ȣ����������ʵ�鷽�������в��ܲⶨNa2CO3��NaHCO3�������Na2CO3�����������ǣ�������
A��ȡa�˻�����ּ��ȣ�����b��
B��ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b�˹���
C��ȡa�˻����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b��
D��ȡa�˻����������Ba��OH��2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b�˹��壮
����A��������ʵ�飬��ԭ�������NaHCO3����������Ϊ$\frac{84b}{31a}$��100%���ú�a��b�Ĵ���ʽ��ʾ���������ܽ�ȱ�
| �¶� �ܽ�� | 10�� | 20�� | 30�� | 40�� |
| NaCO3 | 12.5g | 21.5g | 39.7g | 40.0g |
| NaHCO3 | 8.1g | 9.6g | 11.1g | 12.7g |
| A�� | v��NH3��=0.25 mol•L-1•min-1 | B�� | v��O2��=0.35mol•L-1•min-1 | ||
| C�� | v��NO��=0.01 mol•L-1•s-1 | D�� | v��H2O��=0.3 mol•L-1•min-1 |
 ��-COOH 4�ֻ���������϶��ɵ��л����У��ܸ�NaOH��Һ������ѧ��Ӧ���У�������
��-COOH 4�ֻ���������϶��ɵ��л����У��ܸ�NaOH��Һ������ѧ��Ӧ���У�������| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 6�� |
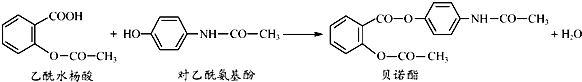
�����й�������ȷ���ǣ�������
| A�� | ��ŵ�������������ֺ��������� | |
| B�� | ����ˮ����Ͷ����������Ӿ�����NaHCO3��Һ��Ӧ | |
| C�� | ����FeCl3��Һ��������ˮ����Ͷ����������� | |
| D�� | ��ŵ��������NaOH��Һ���ȣ�������������ˮ�����ƺͶ������������� |