��Ŀ����
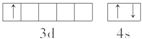
14������Ҫ������������⣺��1��ijԪ��ԭ�ӹ���3���۵��ӣ�����һ���۵���λ�ڵ����ܲ�d������Իش𣺸�Ԫ�غ���۵����Ų�ͼ
 ����Ԫ�ص�ԭ������Ϊ21����Ԫ�ص�Ԫ�ط�����Sc���γɵĵ���Ϊ�������壮
����Ԫ�ص�ԭ������Ϊ21����Ԫ�ص�Ԫ�ط�����Sc���γɵĵ���Ϊ�������壮��2��ָ�������K3[Co��CN��6]�е��������ӵĻ��ϼ�Ϊ+3��
��3�����������ʢ�CO2����NH3����CCl4����BF3����H2O����SO2����SO3����PCl3�У����ڷǼ��Է��ӵ��ǣ����ţ��٢ۢܢߣ�
��4���ԱȽ����к����������ǿ�������������������=������HClO3��HClO4��
��5�����ݼ۲���ӶԻ��������ж��������⣺
��NH3�����У�����ԭ�ӵ��ӻ���ʽΪsp3�ӻ������ӵ����幹��Ϊ�����Σ�
��BF3�����У�����ԭ�ӵ��ӻ���ʽΪsp2�ӻ������ӵ����幹��Ϊƽ�������Σ�
��6��H2O�ķе㣨100�棩��H2S�ķе㣨-61�棩�ߣ���������ˮ����֮������з��»���������������������֮��ֻ�з��»�����
���� ��1��ijԪ��ԭ�ӹ���3���۵��ӣ�����һ���۵���λ�ڵ����ܲ�d������۵����Ų�ʽΪ3d14s2�����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d14s2�����ڵ������ڢ�B�壬Ϊ����Ԫ�أ�
��2�������K3[Co��CN��6]�е���������ΪCo3+��
��3����ͬ�ǽ���Ԫ��֮�����γɼ��Լ������ӽṹ�Գƣ�������������غϵķ���Ϊ�Ǽ��Է��ӣ�
��4��ͬһԪ�صĺ����ᣬ��Ԫ�صĻ��ϼ�Խ�ߣ���Ӧ�ĺ����������Խǿ��
��5����Nԭ�Ӽ۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ�����
��Bԭ�Ӽ۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ�����
��6������ˮ���Ӽ��������Լ����Էе��Ӱ�������
��� �⣺��1��ijԪ��ԭ�ӹ���3���۵��ӣ�����һ���۵���λ�ڵ����ܲ�d������۵����Ų�ʽΪ3d14s2����۵����Ų�ͼΪ �����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d14s2��ԭ������Ϊ21��ΪScԪ�أ����ڵ������ڢ�B�壬���ڽ���Ԫ�أ��γɽ������壬
�����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d14s2��ԭ������Ϊ21��ΪScԪ�أ����ڵ������ڢ�B�壬���ڽ���Ԫ�أ��γɽ������壬
�ʴ�Ϊ�� ��21��Sc��������
��21��Sc��������
��2�������K3[Co��CN��6]�е���������ΪCo3+�����������ӵĻ��ϼ�Ϊ+3�ۣ��ʴ�Ϊ��+3��
��3����CO2��CԪ�ػ��ϼ�Ϊ+4��Cԭ�������4������ȫ���ɼ���Ϊ�Ǽ��Է��ӣ�
��NH3��CԪ�ػ��ϼ�Ϊ-3��Nԭ�������5������δȫ���ɼ���Ϊ���Է��ӣ�
��CCl4��CԪ�ػ��ϼ�Ϊ+4��Cԭ�������4������ȫ���ɼ���Ϊ�Ǽ��Է��ӣ�
��BF3��BԪ�ػ��ϼ�Ϊ+3��Bԭ�������3������ȫ���ɼ���Ϊ�Ǽ��Է��ӣ�
��H2O��OԪ�ػ��ϼ�Ϊ-2��Oԭ�������6������δȫ���ɼ���Ϊ���Է��ӣ�
��SO2��SԪ�ػ��ϼ�Ϊ+4��Sԭ�������6������δȫ���ɼ���Ϊ���Է��ӣ�
��SO3��SԪ�ػ��ϼ�Ϊ+6��Sԭ�������6������ȫ���ɼ���Ϊ�Ǽ��Է��ӣ�
��PCl3��PԪ�ػ��ϼ�Ϊ+3��Pԭ�������5������δȫ���ɼ���Ϊ���Է��ӣ�
��ѡ�٢ۢܢߣ�
��4��ͬһԪ�صĺ����ᣬ��Ԫ�صĻ��ϼ�Խ�ߣ���Ӧ�ĺ����������Խǿ�������ԣ�HClO3��HClO4��
�ʴ�Ϊ������
��5����NH3��������Nԭ�ӹµ��Ӷ���=$\frac{5-1��3}{2}$=1���۲���Ӷ���=3+1=4����Nԭ�Ӳ�ȡsp3�ӻ���VSEPR����Ϊ�������壬���ӵ����幹��Ϊ�����Σ�
�ʴ�Ϊ��sp3�������Σ�
��BF3��������Bԭ�ӹµ��Ӷ���=$\frac{3-3��1}{2}$=0���۲���Ӷ���=3+0=3����Bԭ�Ӳ�ȡsp2�ӻ���VSEPR����Ϊƽ�������Σ����ӵ����幹��Ϊƽ�������Σ�
�ʴ�Ϊ��sp2��ƽ�������Σ�
��6��ˮ����֮������з��»��������������������������ڷ��»��������������֮��ֻ�з��»���������H2O�ķе㣨100�棩��H2S�ķе㣨-61�棩�ߣ�
�ʴ�Ϊ��ˮ����֮������з��»���������������������֮��ֻ�з��»�����
���� ���⿼�������ʽṹ�����ʣ��漰��������Ų������ӽṹ�����ʡ��۲���ӶԻ������۵ȣ���Ŀ�Ѷ��еȣ�ע����ռ۲���ӶԻ������۵�Ӧ�ã�

 �Ķ��쳵ϵ�д�
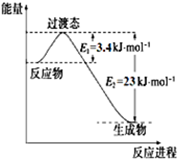
�Ķ��쳵ϵ�д���1���ϳɰ����õ��������Լ���Ϊԭ���Ƶã��йػ�ѧ��Ӧ�������仯��ͼ1��ʾ��
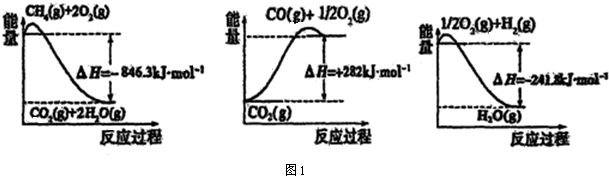
CH4��g����H2O��g����Ӧ����CO��g����H2��g�����Ȼ�ѧ����ʽΪ��CH4��g��+H2O��g��=CO��g��+3H2 ��g����H=+161.1kJ•mol-1��
��2��CO�Ժϳɰ��Ĵ����ж������ã����������������ͭ��Һ������ԭ������CO���䷴Ӧԭ��Ϊ��
[Cu��NH3��2CH3COO]��l��+CO��g��+NH3��g��?[Cu��NH3��3]CH3COO•CO��l������H��0
����CO�������ͭ��Һ�����ʵ��������ֿ��������ָ�������CO�������Թ�ѭ��ʹ�ã�����������������B������дѡ���ţ�
A�����¡���ѹ�� ��B�����¡���ѹ���� C�����¡���ѹ��������D�����¡���ѹ
��3��������ȡ����[CO��NH2��2]�ķ�ӦΪ��2NH3��g��+CO2��g��?CO��NH2��2��1��+H2O��g����H��0��ij�¶��£����ݻ�Ϊ100L���ܱ�������ͨ��4molNH3��2molCO2���÷�Ӧ���е�40sʱ�ﵽƽ�⣬��ʱCO2��ת����Ϊ50%�����¶��´˷�Ӧƽ�ⳣ��KΪ2500L2•mol-2��
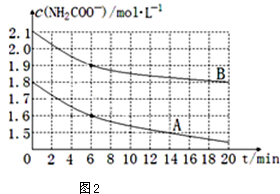
��4��ȡ������ͬ�ĺ���������������ͬ�¶ȣ������������CO2���壬����ʵ�����ݻ��Ƴ�c��NH3����ʱ�䣨t���仯��������ͼ2��ʾ����A��B�ֱ�Ϊ��ͬ�¶�ʱ�ⶨ�����ߣ���A���A����B������������Ӧ��ʵ���¶ȸߣ��жϵ�������A������ʼŨ��С������20minʱ���ڷ�Ӧ���ʿ죬˵�����¶ȸߣ�
��5����֪ijЩ���������ˮ�еĵ���ƽ�ⳣ����25�棩���±���
| ������� | H2CO3 | NH3•H2O |
| ����ƽ�ⳣ�� | Ka1=4.30��10-7��������Ka2=5.61��10-11 | Kb=1.77��10-5 |
�ٸ���Һ�ʼ��ԣ���ᡱ�����С����������ԭ��������NH3•H2O�ĵ���ƽ�ⳣ������HCO3-�ĵ���ƽ�ⳣ�������CO32-ˮ��̶ȴ���NH4+ˮ��̶ȣ���Һ��c��OH-����c��H+������Һ�ʼ��ԣ�
�ڸã�NH4��2CO3��Һ�и���Ũ��֮��Ĺ�ϵʽ����ȷ����B����
A��c��NH4+����c��CO32-����c��HCO3-����c��NH3•H2O��
B��c��NH4+��+c��H+��=c��HCO3-��+c��OH-��+c��CO32-��
C��c��CO32-��+c��HCO3-��+c��H2CO3��=0.1mol•L-1
D��c��NH4+��+c��NH3•H2O��=2c��CO32-��+2c��HCO3-��+2c��H2CO3��
| A�� | �������ӻ����� | B�� | ���ȶ��ֽܷ������ | ||
| C�� | ��������ˮ | D�� | ��������Ӧ�ų����� |
| A�� | Li��Be��Bԭ�������������������� | |
| B�� | P��S��ClԪ����������ϼ��������� | |
| C�� | N��O��Fԭ�Ӱ뾶�������� | |
| D�� | Na��K��Rb�ĵ��Ӳ����������� |
| A�� | ֻ�н���Ԫ�غͷǽ���Ԫ�ػ���ʱ�����γ����Ӽ� | |
| B�� | ���ʷ����о����ڻ�ѧ�� | |
| C�� | �������Ӽ�ͨ�������������γɵĻ�ѧ���������Ӽ� | |
| D�� | ���й��ۼ��Ļ����ﲻһ���ǹ��ۻ����� |
| A�� | ��ɫʯ����Һ | B�� | �Ȼ�����Һ | ||
| C�� | ���Ը��������Һ | D�� | ����ʯ��ˮ |
| A�� | ��CH3��2CHCH2CH2CH3 | B�� | ��CH3��3CCH2CH3 | C�� | ��CH3��2CHCH��CH3��2 | D�� | ��CH3CH2��2CHCH3 |