��Ŀ����
2��������Һ���й��������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | pH=12��Ba��OH��2��Һ��pH=12��Na2CO3��Һ�У�ˮ�����c��OH-����� | |
| B�� | �����½������ơ���������Һ��Ϻ���Һ�����ԣ����Ϻ���Һ�У�c��Na+����c��Cl-����c��CH3COOH�� | |
| C�� | ���������ʵ���Ũ����ȵĢ�NH4HCO3����NH4HSO4����NH4Fe��SO4��2��������Һ��NH4+��Ũ�ȣ��ڣ��ۣ��� | |
| D�� | ����������ʵ���Ũ�ȵ�NaClO��aq����NaCl��aq��������������Nǰ��N�� |
���� A��Ba��OH��2Ϊǿ������ˮ�ĵ��룬Na2C03Ϊǿ��������ˮ��ٽ�ˮ�ĵ��룻
B���������ƣ���������Һ��ϣ�����֮�䷴Ӧ�����Ȼ��ƺʹ��ᣬ��Һ�����ԣ�������ƻ�ʣ�࣬���ݵ���غ�������غ�֪ʶ���ش�
C��̼������Ӵٽ�笠�����ˮ�⡢������������笠�����ˮ�⣬笠�����ˮ��̶�Խ����Һ��c��NH4+��ԽС��
D�����ݵ���غ��֪NaClO��aq����c��Na+��+c��H+��=c��OH-��+c��ClO-�������Դ���������Һ��������Ũ��Ϊ2[c��Na+��+c��H+��]�����ݵ���غ��֪�Ȼ�����Һ��������Ũ��ҲΪ2[c��Na+��+c��H+��]�������Һ��������Ũ�ȴ�С�жϣ�NaClO����ˮ�⣬��Һ�д������ˮ�⣬��Һ�ʼ��ԣ�����c��OH-����c��H+���������ʵ���Ũ�ȵ�NaClO��aq����NaCl��aq���У�����������Һ��������Ũ��С��NaCl��aq����������Ũ�ȣ�
��� �⣺A��ˮ�ĵ��뷽��ʽΪH2O?H++OH-��Ba��OH��2Ϊǿ��ˮ�ĵ��룬Na2C03Ϊǿ�������δٽ�ˮ�ĵ��룬���Na2C03��Һ��ˮ�����c��OH-����A����
B��ԭ��ֻ�д����Ƽ��������û�д��������Ҳû�������ӣ���n��Na+��=n��CH3COOH��+n��CH3COO-������ˣ���C��Na+��=C��CH3COOH��+C��CH3COO-����
��Ϊ�����ԣ�c��H+��=c��OH-���ӵ���غ��֪��C��Na+��+C��H+��=C��CH3COO-��+C��Cl-��+C��OH-�������Ǣ�C��Na+��=C��CH3COO-��+C��Cl-�������C��Na+����C��Cl-����Ȼ��Ѣٴ����ʽ����ȥC��Na+����c��Cl-��=c��CH3COOH������˵ó�C��Na+����C��Cl-��=c��CH3COOH������B����
C����NH4HCO3��NH4+ˮ������ԣ�HCO3-ˮ��ʼ��ԣ����ٽ�NH4+��ˮ�⣻��NH4HSO4��HSO4-��ˮ��Һ����ȫ�������H+������NH4+��ˮ�⣻��NH4Fe��SO4��2�У�Fe3+ˮ������ԣ���Fe3+ˮ�����H+��Ũ��С����������NH4+��ˮ�����Ƴ̶�С������������Һ��c��NH4+�����٣��ۣ��ڣ���C��ȷ��
D�����ݵ���غ��֪NaClO��aq����c��Na+��+c��H+��=c��OH-��+c��ClO-�������Դ���������Һ��������Ũ��Ϊ2[c��Na+��+c��H+��]�����ݵ���غ��֪�Ȼ�����Һ��������Ũ��ҲΪ2[c��Na+��+c��H+��]��NaClO����ˮ�⣬��Һ�д������ˮ�⣬��Һ�ʼ��ԣ�����c��OH-����c��H+���������ʵ���Ũ�ȵ�NaClO��aq����NaCl��aq���У�����������Һ��c��H+��С��NaCl��aq����c��H+��������Һ��c��Na+����ͬ�����ԣ�����������ʵ���Ũ�ȵ�NaClO��aq����NaCl��aq���������������٣�Nǰ��N������D����
��ѡC��
���� ���⿼������Ũ�ȴ�С�Ƚϣ���ȷ���ʵ�����ȷ����Һ����ԣ��ٽ���غ�˼�롢���Ӧ�õ���غ�ʽ�����غ㡢���Ӻ��ʽ�������ע�⣨2������֮����Ӱ�죬��Ŀ�Ѷ��еȣ�

| A�� | Na | B�� | Fe | C�� | O | D�� | Ar |
| A�� | Al2O3�۵�ܸߣ�����������NaOH���������� | |
| B�� | ������ˮ������Al��OH��3���壬��������ˮ�� | |
| C�� | þ�ڿ�����ȼ�շ���ҫ�۵İ⣬���������������� | |
| D�� | ��������Ʒ���渲��������Ĥ�����ڲ������𱣻����� |
| A�� | 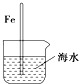 ���뺣ˮ�е�������Խ�����˸�ʴԽ���� | |
| B�� | 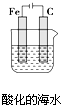 ������Һ�е����������ܽ⣬��Ҫ�Ƿ����绯ѧ��ʴ | |
| C�� |  ȼ��������IJ�λ�������⣬��Ҫ�����ڸ�������������ѧ��ʴ | |
| D�� | 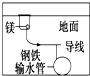 ������þ��ķ�������ֹ���¸����ܵ��ĸ�ʴ��þ���൱��ԭ��ص����� |
| A�� | Fe��Mg��Al����������е�������Ӧ�ڱ����������ܵ�����Ĥ�����ڲ�����б������� | |
| B�� | Fe�ڸ���������ˮ������Ӧ��Fe����������Fe2O3��H2O����ԭ����H2 | |
| C�� | ����Na2CO3��NaHCO3��Һ����������Һ�м���Ba��OH��2��Һ�����������ľ���Na2CO3 | |
| D�� | Al��NaOH��Һ�ķ�Ӧ�У�NaOH�Ȳ����������ֲ��ǻ�ԭ�� |
| A�� | �ж���Ӧ������������������ | |
| B�� | ά����C�ܽ�Fe3+����ΪFe2+��ʹ��ⶾ | |
| C�� | �ж���Ӧ��Fe3+�ǻ�ԭ���� | |
| D�� | ά����C�ܽ��������������������Σ�ʹ��ⶾ |
| A�� | ��1mol CaC2��KHSO4��Na2O2�������ӻ������У��������������ӵ�������Ϊ3NA | |
| B�� | 1molCu��������Ũ���ᷴӦ������nA��SO3���� | |
| C�� | 3mol����Fe��ȫת��ΪFe3O4��ʧȥ8nA������ | |
| D�� | 78gNa2O2������ˮ��Ӧת�Ƶĵ�����Ϊ2NA |
2Fe2++H2O2+2H+�T2Fe3++2H2O 2Fe3++H2O2�T2Fe2++O2��+2H+
����˵����ȷ���ǣ�������
| A�� | H2O2�������Ա�Fe3+ǿ���仹ԭ�Ա�Fe2+�� | |
| B�� | ��H2O2�ֽ�����У���Һ��������ǿ | |
| C�� | H2O2�ֽ���ܷ���ʽΪ��H2O2�TH2O+O2�� | |
| D�� | H2O2��������Ҫ�ϸ�������Fe2+ |

 $\stackrel{����}{��}$
$\stackrel{����}{��}$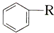 +HCl
+HCl
 ��
�� ��
��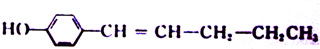 ��
��