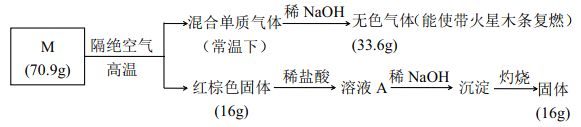
��Ŀ����
����Ŀ����ҵ�ϣ�����ұǦ����(����Pb��PbO��PbS��PbCO3��Pb(OH)2��C�Լ�Fe�����������)��ȡ���·�(ZnS��BaSO4)������ȡǦ�Ĺ����������£�
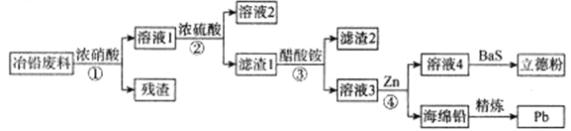
��֪��
�ٴ���Ǧ������ˮ���ѵ��롣
�ڳ����£�Ksp(PbSO4)=1.6��10��8��Ksp(PbCO3)=8.0��10��14��
�ش��������⣺
(1)Ϊ�˼ӿ�ٵķ�Ӧ���ʣ�ͨ��ѡ��6mol/L�������60��ķ�Ӧ���������¶ȼ������ߣ����������ڷ�Ӧ�Ľ��У�ԭ�������___________��
(2)���������ʵ���ҽ��з����������IJ���������___________������1����Ҫ�ɷ�������Ǧ��ϴ������Ǧ���ѡ��___________��
A.����ˮ B.�� C.ϡ���� D.����̼����
(3)д������۷�Ӧ�����ӷ���ʽ___________��
(4)���õ�ⷨ����Ǧ������Ǧ���ӵ�Դ��___________��(��������)������Ǧ�ĵ缫��ӦʽΪ___________��
(5)������Ǧ����1t(��Ǧ��PbO�ƣ���������Ϊ11.15%)����������Ϊ90%����õ���Ǧ����___________g��
���𰸡��¶ȹ��ߣ������ֽ� ©�����ձ��������� C PbSO4��2CH3COO��=(CH3COO)2Pb��SO42�� ���� Pb2����2e��=Pb 9.315��104
��������
��1��ͨ��ѡ��6mol/L�������60��ķ�Ӧ�������¶ȹ����������ֽ⡣
��2������ܷ�Ӧ��ķ�������ǹ��ˣ�ѡ©�����ձ�����������ϴ������ǦҪϴȥ�������������ʣ���Ҫ������ʧ�����ѡ��ϡ���ᡣ
��3������۷�Ӧ�����ӷ���ʽPbSO4��CH3COO����������������ˮ���ѵ����(CH3COO)2Pb��
��4�����õ�ⷨ����Ǧ������Ǧ���ӵ�Դ��������ǦԪ�ػ��ϼ����ߣ�����Ǧ�ĵ缫��Ӧʽ��Pb2����2e��=Pb
��5������Ǧ�غ���㡣
��1�����������ֽ������¶Ȳ���̫����
��2������ܷ�Ӧ��ķ������Ϊ��������������IJ������������ձ���©���Ͳ�������ϴ������ǦҪϴȥ������������������Ҫ������ʧ����A�����������Ǧ�����ܽ���B.���������Ӳ����ܽ�������ϴȥ��C��ϡ�����ܹ�ϴȥ������ͬʱ�ɼ�������Ǧ���ܽ���D��������������Ǧ��ת��Ϊ̼��Ǧ���������ʡ���ѡC��
��3�����������Ǧ������ˮ���ѵ������ʷ�Ӧ�����ӷ���ʽΪPbSO4��2CH3COO��=(CH3COO)2Pb��SO42����
��4�����õ�ⷨ����Ǧ������Ǧ���ӵ�Դ��������ǦԪ�ػ��ϼ����ߣ�����Ǧ�ĵ缫��Ӧʽ��Pb2����2e��=Pb��
��5������ǦԪ���غ�ɵ�Ϊ![]() =9.315��104g��
=9.315��104g��