��Ŀ����
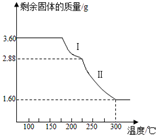
9��̼����һ����Ҫ�Ļ���ԭ�ϣ���ҵ̼���ƴ���ԼΪ 98%�����к��� Ca2+��Mg2+��Fe3+��Cl-�� SO42-�����ʣ��ᴿ������·��ͼ��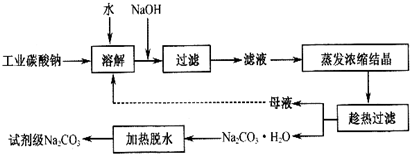
�й����ʵ��ܶȻ������
| ���� | CaCO3 | MgCO3 | Ca��OH��2 | Mg��OH��2 | Fe��OH��3 |
| Ksp | 4.96��10-9 | 6.82��10-6 | 4.68��10-6 | 5.61��10-12 | 2.64��10-39 |
��1��̼������������ͨ��������Ҫԭ�ϣ���д��������ͨ���������з����Ļ�ѧ��Ӧ����ʽSiO2+Na2CO3$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2����
��2�������ᴿ�����м���NaOH��Һʱ����Ӧ�����ӷ���ʽΪFe3++3OH-=Fe��OH��3����MgCO3+2OH-=Mg��OH ��2+CO32-������Һ��pH=8ʱ����Һ��c��Mg 2+����c��Fe3+��=2.125��1021��
��3����ĸҺ���г��˺��� Na+��CO32-�����⣬������Cl-��SO42-�����ӣ�
��4�����˴ӡ���ɫ��ѧ���Ƕ����뽫��ĸҺ��ֱ�ӽ���ѭ��ʹ�ã��������ʵ�ʹ�ҵ�������Ƿ���в����У�����С������С�����˵����������ĸҺ��ѭ��ʹ�ã�����Һ�� c��Cl-����c��SO42-������������ò���
Na2 CO3�������ʣ�
��5���Լ���̼���Ƶļ����Ϊ�����ȡ�99.5%��Ϊ�˼����ᴿ���̼�����Ƿ��꣬ʵ���ҳ�ȡ1.06g��Ʒ������ˮ���Ƴ�100mL��Һ����ȡ20.00mL������2�μ�����Ϊָʾ������0.1000mol/L�ı�������еζ������εζ���ʹ�������ƽ�����Ϊ39.84mL������㣬����Ʒ̼���ƵĴ���Ϊ99.6%��
���� ��1��������ͨ���������У�̼������������跴Ӧ���ɹ����ƺͶ�����̼��
��2����ҵ̼�����к���Mg2+��Fe3+��Ca2+�����ԡ����ӡ��м��������NaOH��Һ��������Mg��OH��2��Fe��OH��3��CaCO3������
��3����ȥg2+��Fe3+��Ca2+����Һ�г��˺��� Na+��CO32-�����⣬������Cl-��SO42-��
��4������ĸҺ��ѭ��ʹ�ã�����Һc��Cl-����c��SO42-������������ò���Na2CO3�л������ʣ�
��5��������ζ�̼����Ӧѡ�ü�����ָʾ�����ζ��յ��ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ�����ݹ�ϵʽNa2CO3��2HCl������̼���ƵĴ��ȣ�
��� �⣺��1��������ͨ���������У�̼������������跴Ӧ���ɹ����ƺͶ�����̼����ѧ����ʽΪSiO2+Na2CO3$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2����
�ʴ�Ϊ��SiO2+Na2CO3$\frac{\underline{\;����\;}}{\;}$Na2SiO3+CO2����
��2��̼�����к���Mg2+��Fe3+��Ca2+�����ԡ����ӡ��м��������NaOH��Һ��������Mg��OH��2��Fe��OH��3��CaCO3��������������Ҫ�ɷ�ΪMg��OH��2��Fe��OH��3��CaCO3�������ķ�Ӧ�У�Fe3++3OH-=Fe��OH��3����MgCO3+2OH-=Mg ��OH�� 2��+CO32-������������þ�������������ܽ�ƽ���Լ��ܶȻ�����ʽ�������ֳ�����������Һ��pH=8ʱ��c��Mg2+��=$\frac{Ksp}{{c}^{2}��O{H}^{-}��}$=$\frac{5.16��1{0}^{-12}}{��1{0}^{-6}��^{2}}$=5.61mol/L��c��Fe3+��=$\frac{2.64��1{0}^{-39}}{��{1{0}^{-6}��}^{3}}$=2.64��10-21mol/L��
���� c��Mg2+����c��Fe3+��=5.61��2��.64��10-21=2.215��1021��
�ʴ�Ϊ��Fe3++3OH-=Fe��OH��3����MgCO3+2OH-=Mg��OH ��2��+CO32-�� 2.215��1021��
��3��̼�����к���Mg2+��Fe3+��Ca2+�����ԡ����ӡ��м��������NaOH��Һ��������Mg��OH��2��Fe��OH��3��CaCO3���������˺���Һ�г��˺��� Na+��CO32-�����⣬������Cl-��SO42-��
�ʴ�Ϊ��Cl-��SO42-��
��4������ĸҺ��ѭ��ʹ�ã�����Һc��Cl-����c��SO42-������������ò���Na2CO3�л������ʣ����������ϸ��ᴿ���գ�
�ʴ�Ϊ�������У�����ĸҺ��ѭ��ʹ�ã�����Һc��Cl-����c��SO42-������������ò���Na2CO3�л������ʣ�
��5��������ζ�̼����Ӧѡ�ü�����ָʾ�����ζ��յ��ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ��
���ݹ�ϵʽNa2CO3��2HCl��
1mol 2mol
n 0.1000mol/L��0.03984L��n=0.001992mol��
�����Ʒ̼���ƵĴ���Ϊ$\frac{0.001992mol��106g/mol��5}{1.06g}$��100%=99.6%��
�ʴ�Ϊ�����ȣ� 99.6%��
���� ���⿼�������ʵķ��롢�ᴿ�����ʵ���ȡ��֪ʶ����Ŀ�Ѷ��еȣ�������һ���Ƚ��ۺϵ������⣬�����ڿ���ѧ�������ͽ�������������

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�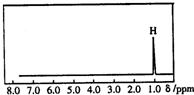 �˴Ź������������л����е�1H��ԭ�Ӻ������ġ���ѧ�����������丽���Ļ��ţ���ͬ�����ֳ��ĺ˴��Բ�ͬ�������˴��������ķ��ں˴Ź�����ͼ�к������λ�û�ѧλ�ƣ�����Ϊ�ģ�Ҳ�Ͳ�ͬ����ͼ��ʾ�ĺ˴Ź���ͼ�ױ�ʾ���������л����еģ�������
�˴Ź������������л����е�1H��ԭ�Ӻ������ġ���ѧ�����������丽���Ļ��ţ���ͬ�����ֳ��ĺ˴��Բ�ͬ�������˴��������ķ��ں˴Ź�����ͼ�к������λ�û�ѧλ�ƣ�����Ϊ�ģ�Ҳ�Ͳ�ͬ����ͼ��ʾ�ĺ˴Ź���ͼ�ױ�ʾ���������л����еģ�������| A�� | CH3C��CH3��3 | B�� | CH3CH2CH3 | C�� | CH2=CHCH3 | D�� | ��CH3��2CHCH3 |
| A�� | �Ҵ���������Ũ������������·�Ӧ | |
| B�� | ��ϩͨ��������Ȼ�̼��Һ | |
| C�� | ����Ũ���ᡢŨ����Ļ���ﹲ�� | |
| D�� | �Ҵ���ͭ�۴�������������Ӧ |
| A�� | 9�� | B�� | 16�� | C�� | 19�� | D�� | 22�� |
| A�� | ȩ���Ľṹ��ʽ-COH | B�� | �۱�ϩ�Ľṹ��ʽ | ||
| C�� | �ǻ��ĵ���ʽ  | D�� | �Ҵ��Ľṹ��ʽC2H6O |
| A�� | ������202 | B�� | ���������202 | C�� | ԭ������78 | D�� | ������124������ |
| A�� | ���ϵ�����ɷ�ֹ�ؽ�����Ⱦ | |
| B�� | �����Է����еĻ�ѧ��Ӧ���Ƿ��ȷ�Ӧ | |
| C�� | ����̫���ܡ����ܺ����ܵ���Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ���������� | |
| D�� | �������ÿ�ȼ���ǻ�����Դ��ȱ��Ψһ;�� |

 ����
���� ����ԭ�ӽ���ˮ�⣮
����ԭ�ӽ���ˮ�⣮ ��
�� ��
�� ��
��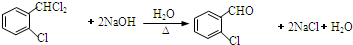 ��
�� ��
�� ��
�� ��д������ϩ���״�Ϊ�л�ԭ���Ʊ�������
��д������ϩ���״�Ϊ�л�ԭ���Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2$\stackrel{Br_{2}}{��}$
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2$\stackrel{Br_{2}}{��}$