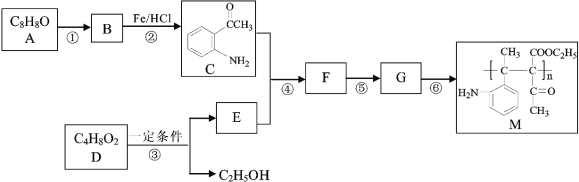
��Ŀ����
����Ŀ��Ϊ��ʹ�Ա�ڷɴ��еõ�һ���ȶ��ġ����õ����滷����һ���ڷɴ��ڰ�װʢ��Na2O2��K2O2������װ�ã�������;�Dz������������й���Na2O2��������ȷ���ǣ� ��
��Na2O2�����������ӵĸ�������1��1
��Na2O2�ֱ���ˮ��CO2��Ӧ������������ʱ��ת�Ƶ��ӵ����ʵ������
��Na2O2Ͷ�뵽��ɫʯ����Һ�У���Һ�ȱ���������ɫ
��Na2O2�����ᷴӦ�����κ�ˮ������Na2O2�Ǽ���������
��Na2O2��ˮ��Ӧ��Na2O2���������������ǻ�ԭ��
A.�٢ۢ�B.�ڢۢ�C.�٢ڢ�D.�٢ܢ�
���𰸡�B
��������
��Na2O2�ĵ���ʽΪ![]() �������������ӵĸ�����Ϊ1��2������
�������������ӵĸ�����Ϊ1��2������
�ڷ�Ӧ�ķ���ʽ�ֱ�Ϊ2Na2O2+2CO2=2Na2CO3+O2��2Na2O2+2H2O=4NaOH+O2�����ɵ�ʧ�����غ�֪����ת�Ƶ��ӵ����ʵ�����ͬ����ȷ��
��Na2O2Ͷ�뵽��ɫʯ����Һ�У���ˮ��Ӧ�����������Ƴɼ���������Һ�ȱ���������Ϊ�������ƾ���ǿ�����Զ���Ư���ԣ����Ժ���ɫ����ȷ��
��Na2O2�ܺ����ᷴӦ�����Ȼ��ƺ�ˮ��������Ϊ����������Ǽ������������
��Na2O2��ˮ��Ӧ��ֻ��OԪ�ػ��ϼ۷����仯����-1�۷ֱ�仯Ϊ0��-2�ۣ�Na2O2���������������ǻ�ԭ������ȷ��
��ѡB��

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�����Ŀ���й���ѧ���ý����ƺ�![]() ��һ���������Ƶ��˽��ʯ��
��һ���������Ƶ��˽��ʯ��![]() �����ʯ��
�����ʯ��
�� |
| ���ʯ | ʯī | |
�۵㣨�棩 | 97.8 | 851 | 3550 | 3850 |
�е㣨�棩 | 882.9 | 1850���ֽ���� | ���� | 4250 |
(1)����Ӧ�ڳ�ѹ��890���½��У�д���÷�Ӧ��ƽ�ⳣ������ʽ____����![]() ����________��ѡ����ţ���
����________��ѡ����ţ���
a.��Ӧ�϶��ﵽƽ�� b.��Ӧ���ܴﵽƽ�� c.��Ӧ�϶�δ��ƽ��
(2)����Ӧ��10L�ܱ���������ѹ�½��У�5min�ڣ���ý��ʯ������������6g����ʱ�����![]() ________������Ӧ�¶���890�����ߵ�1860�棬�������������ƽ����Է���������________��ѡ����������������С����������������
________������Ӧ�¶���890�����ߵ�1860�棬�������������ƽ����Է���������________��ѡ����������������С����������������
(3)��Ӧ�л���ʯī���ɣ���֪��![]() ���������¶ȣ����ɵ�̼�����У����ʯ�ĺ�����������÷�Ӧ������Ӧ��________��Ӧ��������������������������
���������¶ȣ����ɵ�̼�����У����ʯ�ĺ�����������÷�Ӧ������Ӧ��________��Ӧ��������������������������