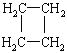��Ŀ����
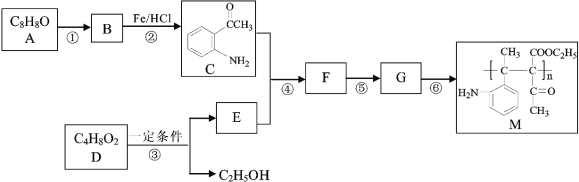
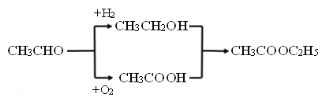
����Ŀ���߷��ӻ�����M�ĺϳ�·�����£�
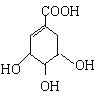
��֪��![]()
(1)A�к��������ŵ�������______��
(2)H��A��ͬ���칹�壬����FeCl3��ʾ�������ɫ���˴Ź���������ʾH��5��壬����ṹ��ʽΪ____________________��
(3) B�Ľṹ��ʽΪ______��G�Ľṹ��ʽΪ__________________��
(4) ��֪DΪ����������2 D �� E + C2H5OH��F�к��д��ǻ����ݵķ�Ӧ����Ϊ_____��
(5) д���ܵķ�Ӧ����ʽ___________________________________________��
(6) ����ȩΪԭ�ϣ�д���ϳ�D������ͼ______��(�������Լ���ѡ)��
���𰸡��ʻ� ![]()
 ��ȥ��Ӧ
��ȥ��Ӧ  +
+ 
![]()
��������
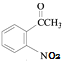
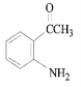
������Ϣ����Ӧ�ڷ�����ԭ��Ӧ����NO2����NH2����B�Ľṹ��ʽΪ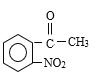 ����Ӧ�ٷ���������Ӧ����A�Ľṹ��ʽΪ
����Ӧ�ٷ���������Ӧ����A�Ľṹ��ʽΪ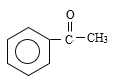 ������M�Ľṹ��ʽ���Ƴ���Ӧ������Ϊ�Ӿ۷�Ӧ����G�Ľṹ��ʽΪ
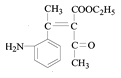
������M�Ľṹ��ʽ���Ƴ���Ӧ������Ϊ�Ӿ۷�Ӧ����G�Ľṹ��ʽΪ ���������⣨4�����Ƴ�E�ķ���ʽΪC6H10O3������G�Ľṹ��ʽ���Ƴ�E�Ľṹ��ʽΪ
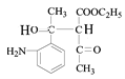
���������⣨4�����Ƴ�E�ķ���ʽΪC6H10O3������G�Ľṹ��ʽ���Ƴ�E�Ľṹ��ʽΪ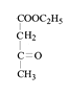 ��F�к��д��ǻ�����Ӧ��Ϊ�ӳɷ�Ӧ���Ƴ�F�Ľṹ��ʽΪ
��F�к��д��ǻ�����Ӧ��Ϊ�ӳɷ�Ӧ���Ƴ�F�Ľṹ��ʽΪ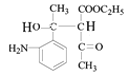 ����Ӧ��������ȥ��Ӧ��
����Ӧ��������ȥ��Ӧ��
������Ϣ����Ӧ�ڷ�����ԭ��Ӧ����NO2����NH2����B�Ľṹ��ʽΪ ����Ӧ�ٷ���������Ӧ����A�Ľṹ��ʽΪ
����Ӧ�ٷ���������Ӧ����A�Ľṹ��ʽΪ ������M�Ľṹ��ʽ���Ƴ���Ӧ������Ϊ�Ӿ۷�Ӧ����G�Ľṹ��ʽΪ
������M�Ľṹ��ʽ���Ƴ���Ӧ������Ϊ�Ӿ۷�Ӧ����G�Ľṹ��ʽΪ ���������⣨4�����Ƴ�E�ķ���ʽΪC6H10O3������G�Ľṹ��ʽ���Ƴ�E�Ľṹ��ʽΪ
���������⣨4�����Ƴ�E�ķ���ʽΪC6H10O3������G�Ľṹ��ʽ���Ƴ�E�Ľṹ��ʽΪ ��F�к��д��ǻ�����Ӧ��Ϊ�ӳɷ�Ӧ���Ƴ�F�Ľṹ��ʽΪ
��F�к��д��ǻ�����Ӧ��Ϊ�ӳɷ�Ӧ���Ƴ�F�Ľṹ��ʽΪ ����Ӧ��������ȥ��Ӧ��
����Ӧ��������ȥ��Ӧ��
��1����������������A�к������������ʻ���
��2������FeCl3������ɫ��Ӧ��˵�����ǻ���H��Ӧ����̼̼˫�����˴Ź���������5��壬˵����5�ֲ�ͬ�������⣬Ӧ�ǶԳƽṹ�����ṹ��ʽΪ![]() ��
��
��3����������������B�Ľṹ��ʽΪ ��G�Ľṹ��ʽΪ
��G�Ľṹ��ʽΪ ��
��
��4��F��G������OH������̼̼˫��������Ӧ�ݵ�����Ϊ��ȥ��Ӧ��
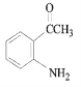
��5����Ӧ��Ϊ�ӳɷ�Ӧ���䷴Ӧ����ʽΪ +
+ 
![]()
 ��
��
��6��DΪ�����������ṹ��ʽΪCH3COOCH2CH3������ȩ���������ᣬ����ȩ��H2�����ӳɷ�Ӧ�����Ҵ���Ȼ��������Ҵ�����������Ӧ���õ���������������ͼΪ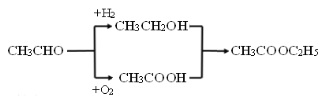 ��
��

����Ŀ����E(g)��F(g)�����ܱ������У���һ�������·�����Ӧ��E(s)��4F(g)G(g)����֪�÷�Ӧ��ƽ�ⳣ�������ʾ������˵����ȷ����(����)
�¶�/�� | 25 | 80 | 230 |
ƽ�ⳣ��/(L3��mol��3) | 5��104 | 2 | 1.9��10��5 |
A.������Ӧ��������Ӧ
B.25 ��ʱ����ӦG(g)E(s)��4F(g)��ƽ�ⳣ����0.5 mol3��L��3
C.��80 ��ʱ�����ijʱ�̣�F��G��Ũ�Ⱦ�Ϊ0.5 mol��L��1�����ʱv����v��
D.���º����£����������ٳ�������G(g)������ƽ��ʱ��G������ٷֺ���������