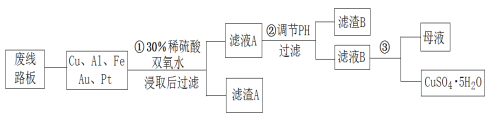
��Ŀ����
����Ŀ���ܵĻ������ڹ�ҵ�����������Ƽ�����ҵ����ҪӦ�á�
(1)Co2+�ĺ�������Ų�ʽΪ___��Co�ĵ��ĵ����ܱ�Fe�ĵ��ĵ�����ҪС�ö࣬ԭ����___��
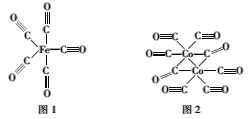
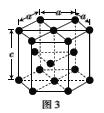
(2)Fe��Co������CO�γ�������Fe(CO)5��Co2(CO)8�Ľṹ��ͼ1��ͼ2��ʾ��ͼ1��1molFe(CO)5����__mol��λ����ͼ2��Cԭ�ӵ��ӻ���ʽΪ___���γ��������ֻ����������Ԫ���е縺��������___����Ԫ�ط��ţ���
(3)�����ܵĶѻ���ʽΪ�������ܶѻ�������λ����___���ܾ��徧���ṹ��ͼ3��ʾ���þ�����ԭ�Ӹ���Ϊ___���þ����ı߳�Ϊanm����Ϊcnm���þ������ܶ�Ϊ___��NA��ʾ�����ӵ�������ֵ���г�����ʽ��g��cm-3��
���𰸡�[Ar]3d7��1s22s22p63s23p63d7 Coʧȥ3�����Ӻ����[Ar]3d6����������ʧȥһ�������γɰ���״̬[Ar]3d5��Feʧȥ�������Ӻ����[Ar]3d5���ﵽ��������ȶ�״̬��������ʧȥһ������ 10 sp��sp2 O 12 6 ![]()
��������
(1)Co2+�ĺ��������Ϊ25�������Ų�ʽΪ[Ar]3d7��1s22s22p63s23p63d7��Co�ĵ��ĵ����ܱ�Fe�ĵ��ĵ�����ҪС�ö࣬ԭ����Coʧȥ3�����Ӻ����[Ar]3d6����������ʧȥһ�������γɰ���״̬[Ar]3d5��Feʧȥ�������Ӻ����[Ar]3d5���ﵽ��������ȶ�״̬��������ʧȥһ�����ӡ���Ϊ��[Ar]3d7��1s22s22p63s23p63d7��Coʧȥ3�����Ӻ����[Ar]3d6����������ʧȥһ�������γɰ���״̬[Ar]3d5��Feʧȥ�������Ӻ����[Ar]3d5���ﵽ��������ȶ�״̬��������ʧȥһ�����ӣ�
(2)��Fe(CO)5�У�ÿ��CO�����У�C��Oԭ�Ӽ��γɵ�3�����ۼ��У���1������λ�������⣬ÿ��Cԭ��������ԭ�Ӽ��γ�1����λ��������1molFe(CO)5����10mol��λ����ͼ2�У�ֻ��1��Coԭ���γ���λ����Cԭ�ӣ��۲���Ӷ���Ϊ2��Cԭ�ӷ���sp�ӻ�����2��Coԭ���γ���λ����Cԭ�ӣ��۲���Ӷ���Ϊ3��Cԭ�ӷ���sp2�ӻ����γ��������ֻ����������Ԫ���зǽ�������ǿ��Ԫ����������縺��������O����Ϊ��10��sp��sp2��O��
(3)�����Ϸ���֪�������ܵĶѻ���ʽΪ�������ܶѻ�������λ����12���þ�����ԭ�Ӹ���Ϊ12��![]() +2��
+2��![]() +3=6���þ����ı߳�Ϊanm����Ϊcnm�����������V=
+3=6���þ����ı߳�Ϊanm����Ϊcnm�����������V=![]() =
=![]() ���þ������ܶ�Ϊ
���þ������ܶ�Ϊ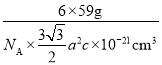 =
=![]() g��cm-3������12��6��
g��cm-3������12��6��![]() ��
��

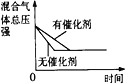
 ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�����Ŀ��������Դ�Ŀ��������þ��й�����ǰ������ˮ��pHһ����7.5~8.6֮�䡣ij�غ�ˮ����Ҫ���ӵĺ������±���
�ɷ� | Na+ | K+ | Ca2+ | Mg2+ | Cl�� | SO42�� | HCO3�� |
����/mgL��1 | 9360 | 83 | 160 | 1100 | 16000 | 1200 | 118 |
��1����ˮ�������Ե�ԭ���ǣ������ӷ���ʽ��ʾ����____________���ú�ˮ��Ca2+�����ʵ���Ũ��Ϊ__________mol/L ��
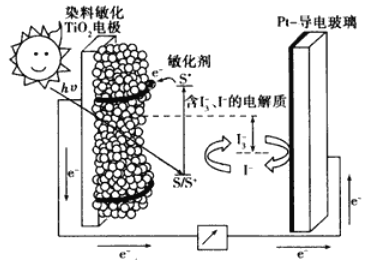
��2�����������ǽ��귢չ������һ�ֽϺõĺ�ˮ������������ԭ������ͼ��ʾ�����������������ӽ���Ĥֻ����������������ͨ�����缫��Ϊ���Ե缫��

�� ��ʼʱ�����ĵ缫��ӦʽΪ________________��
�� ���һ��ʱ�䣬____���������������������������ˮ������ɷ�Ϊ_____���ѧʽ����
�� ��ˮ�ij���Ϊa��b��c�е�__________���ڡ�
��3����ˮ���Ԫ�ش����dz��ḻ���Ӻ�ˮ����ȡ﮵��о�����DZ����������컯ѧ��Դ����Ҫԭ�ϣ���LiFePO4���ij�缫�Ĺ���ԭ����ͼ��ʾ��

�õ�ص����Ϊ�ܴ��� Li+�Ĺ�����ϡ�
������ͼ�е�С�ڵ��ʾ_____�������ӷ��ţ������ʱ�õ缫��ӦʽΪ_______��
��4�����ú�����Դ�ɻ��MnO2 ��MnO2�������Ʊ�������أ���MnO2��KOH��Ϻ��ڿ����м������ڣ��õ���ɫ������أ�K2MnO4��������������������������ɸ�����ء����Ʊ�������������ͬ�����������Ϳ����������Ϊ_________�����������������������20%�ƣ���
����Ŀ������ʵ�ˮ��Һ�д��ڵ���ƽ�⡣
��.��1�������dz��������ᡣ���з����У�����ʹ����ϡ��Һ��CH3COOH����̶��������_____������ĸ��ţ���
a �μ�����Ũ���� b ����Һ c ��ˮϡ�� d �������������ƾ���
�״��dz����������ζ���ϣ��״�����Ȳⶨ�������¡�
a ��ȡ20.00 mL�״���Ʒ����100 mL����ƿ���Ƴɴ���Һ��
b ���ζ���ϴ������ϴ��װ����Һ���ϳ����촦���ݣ�����Һ����0�̶��ߡ�
c ȡ20.00 mL���ƵĴ���Һ�ڽྻ����ƿ�У���3�η�̪��Һ����0.1000 mol�� L��1��NaOH��Һ�ζ����յ㣬��¼���ݡ�
d �ظ��ζ�ʵ��3�β���¼���ݡ�
e ����״���Ʒ�д�������ȡ��ش��������⣺
��2��ʵ��a����ȡ20.00 mL�״����õ�����������___________��
��3����ʵ��b�м�ʽ�ζ���δ��NaOH����Һ��ϴ������ɲⶨ�����ȷֵ_________������ƫ��������ƫС����������������
��4��ʵ��C���жϵζ��յ��������_______��
��5��ʵ���������±�����ð״���Ʒ�д��������Ϊ_________ mol�� L��1��
����Һ���/mL | ��NaOH��Һ | ||
�ζ�ǰ����/mL | �ζ��յ����/mL | ||
��1�� | 20.00 | 0 | 21.98 |
��2�� | 20.00 | 0 | 22.00 |
��3�� | 20.00 | 0 | 22.02 |
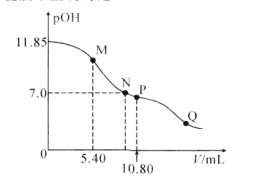
��.25�棬��20.00 mL 0.100 0 mol��L1 CH3COOH�еμ�0.100 0 mol��L��1 NaOH�����У�pH�仯����ͼ��ʾ��
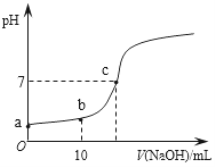
��6��a����ҺpH �� 1���õ��뷽��ʽ����ԭ��_____________��
��7�������й�b����Һ��˵����ȷ����_______������ĸ��ţ���
a ������CH3COOH��CH3COONa
b ��Ũ�����㣺c��Na���� + c��H���� = c��CH3COO���� + c��OH��
c ��Ũ�����㣺c��Na���� = c��CH3COOH�� + c��CH3COO����
d ��Ũ�����㣺2c��H���� + c��CH3COO���� =2c��OH���� +c��CH3COOH��
��8��c����Һ������Ũ�ȵĴ�С˳��__________��
����Ŀ����֪��[FeCl4(H2O)2]-�ʻ�ɫ��������ʵ�����ý��۲���ȷ���ǣ� ��
�� | �� | �� | �� |
0.1mol/L Fe2(SO4)3��Һ |
�ữ��0.1mol/L Fe2(SO4)3��Һ |
�ữ��0.1mol/L Fe2(SO4)3��Һ |
0.1mol/L FeCl3��Һ |
����ǰ��ҺΪdz��ɫ�����Ⱥ���ɫ���� | ����ǰ��Һ�ӽ���ɫ�����Ⱥ���Һ��ɫ�����Ա仯 | ����NaCl����Һ������Ϊ��ɫ�����Ⱥ���Һ��ɫ���� | ����ǰ��ҺΪ��ɫ�����Ⱥ���Һ��ɫ���� |
ע������Ϊ�ȣ�������Һ����仯��
A.ʵ����У�Fe2(SO4)3��Һ��dz��ɫ��ԭ����Fe3+ˮ�����������Fe(OH)3
B.ʵ����У��ữ��Fe3+ˮ���Ӱ��̶ȴ����¶ȵ�Ӱ��
C.ʵ����У�����ƽ�⣺Fe3+ +4Cl- +2H2O![]() [FeCl4(H2O)2]-
[FeCl4(H2O)2]-
D.ʵ����У���֤�������¶ȣ�Fe3+ˮ��ƽ��һ���������ƶ�