��Ŀ����
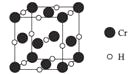
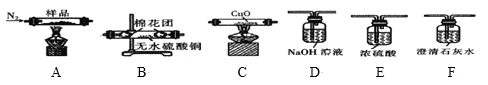
����Ŀ����Ϣʱ�������Ĵ������������Ի��������˼������в�����Խ�����Ϊ��������������·����飬����������Եõ���ͭ��75%Cu��20%Al��4%Fe������Au��Pt������һ����ȡ���������̼�ͼ���£�
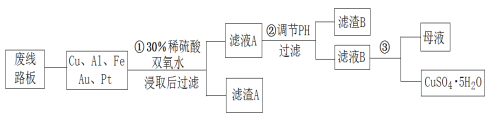
��1������A�ijɷ���Ҫ��_____________��
��2����������ܽ�Cu�Ļ�ѧ����ʽ��_________________����______���˫��ˮ�����ȣ�ͬ�����ԴﵽĿ�ġ�
��3������ڵ�����ҺpH��Ŀ����__________����ѡ�õ�����Լ���___����д��һ�ּ��ɣ�
��4����ʯī���缫�����ҺB����ⷽ��ʽ��___________________��
��5�������ϴ�ͭ���е�⾫��������˵����ȷ����_________��
A.����ȫ��ת��Ϊ��ѧ��
B.��ͭ�ӵ�Դ����������������Ӧ
C.��Һ��Cu2+��Ũ�ȱ��ֲ���
D.�������пɻ���Pt��Au�Ƚ���
��6��ȡ128Kg��ͭ�������������Ƶ�CuSO4��5H2O������Ϊ326.25Kg,��CuSO4��5H2O�IJ���Ϊ__________��
���𰸡�Au Pt Cu+H2O2+H2SO4![]() CuSO4+2H2O O2 ��ȥFe3+��Al3+ CuO��Cu(OH)2��Cu2(OH)2CO3��CuCO3�� 2CuSO4+2H2O
CuSO4+2H2O O2 ��ȥFe3+��Al3+ CuO��Cu(OH)2��Cu2(OH)2CO3��CuCO3�� 2CuSO4+2H2O![]() 2H2SO4+2Cu+O2�� BD 87�� ����86.9%��
2H2SO4+2Cu+O2�� BD 87�� ����86.9%��
��������
��ϡ�����˫��ˮ�Ļ��Һ�ܽ��ͭ������Cu��Al��Fe������Ӧ����Cu2+��Al3+��Fe3+����Au��Pt����Ӧ����������A �ijɷ���Pt��Au����ҺA�е�������Cu2+��Al3+��Fe3+���ڢڲ�������ҺpH��Ŀ����ʹFe3+��Al3+�γɳ�����������ҺB����Ҫ�ɷ�������ͭ���������ᾧ�ɵõ�CuSO45H2O���塣
(1)���ݷ�����֪������A����Ҫ�ɷ�ΪAu ��Pt��
(2)�����ữ��˫��ˮ�ܽ�ͭ����������ͭ��ˮ��������Ӧ�Ļ�ѧ����ʽΪCu+H2O2+H2SO4![]() CuSO4+2H2O��������ǿ�����ԣ����ữ������Ҳ���ܽ�Cu���һ�ԭ����Ϊˮ���������µ����ʣ������˫��ˮ��
CuSO4+2H2O��������ǿ�����ԣ����ữ������Ҳ���ܽ�Cu���һ�ԭ����Ϊˮ���������µ����ʣ������˫��ˮ��
(3)������ҺpH�����Ӻ�������ȫ����������˵õ���������������������������Һ����ͭ���ڲ��������ʵ�ǰ���£���ѡ��CuO��Cu(OH)2��������ҺpH��
(4)��ʯī�缫���CuSO4��Һ��������Cu�������������������ɣ����ⷴӦ�Ļ�ѧ����ʽΪ��2CuSO4+2H2O 2H2SO4+2Cu+O2����
2H2SO4+2Cu+O2����
(5)��⾫��ͭʱ�����˵��ԭ��������ת��Ϊ��ѧ�ܣ�Ҳ������ת��Ϊ���ܡ���⾫��ʱ��ͭ������������������Ӧ����ͭ�������������Ϸ�����ԭ��Ӧ�����ʱ����Һ�е��������������ƶ����������ϵõ��ӡ���ͭ�еIJ����ý�������ʧ���ӣ������������ʽ����������������ѡ��B��D�������⣻
(6)128Kg��ͭ����CuΪ128Kg��75%=96kg�������ʵ���Ϊ![]() =1500mol������ԭ���غ㣬��������CuSO45H2O�����ʵ���Ϊ1500mol��������Ϊ1500mol��250g/mol=375000g=375Kg����CuSO45H2O�IJ���=
=1500mol������ԭ���غ㣬��������CuSO45H2O�����ʵ���Ϊ1500mol��������Ϊ1500mol��250g/mol=375000g=375Kg����CuSO45H2O�IJ���=![]() ��100%=
��100%=![]() ��100%=87%��
��100%=87%��

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�