��Ŀ����
����Ŀ����ͼ��һ��Ⱦ������̫���ܵ�ص�ʾ��ͼ����ص�һ���缫���л�����Ⱦ��(S)Ϳ����TiO2����������Ƴɣ���һ�缫�ɵ��粣���Ʋ����ɣ�����з����ķ�ӦΪ��
TiO2/S��TiO2/S��(����̬)
TiO2/S����TiO2/S��+e-
I3-+2e-��3I-
2TiO2/S++3I-��2TiO2/S+I3-

���й��ڸõ�������������
A. ��ع���ʱ���ǽ�̫����ת��Ϊ����
B. ��ع���ʱ��I-�����ڶƲ����粣���缫�Ϸŵ�
C. ����жƲ����粣��Ϊ����
D. ��صĵ������Һ��I-��I3-��Ũ�Ȳ������
���𰸡�B
����������ͼ�е��ӵ��ƶ������֪��TiO2�缫Ϊ������Pt���粣��Ϊ������I3-��I-��Ԫ�ػ��ϼ۽��ͣ�Ϊ�õ��ӹ�����I-��I3-Ϊʧ���ӹ��̡�A����ͼ��֪�õ�ؽ�̫����ת��Ϊ���ܣ���A��ȷ��B��Pt���粣��Ϊ�����������õ��ӣ�ӦΪI3-��I-����B����C��Pt���粣��Ϊ��������C��ȷ��D���õ����I3-��I-�ת������û�б���ģ���D��ȷ����ѡB��

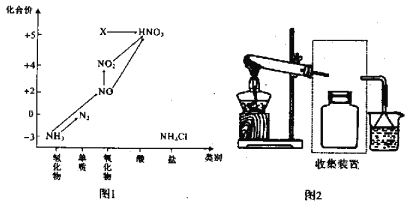
����Ŀ���̷�(FeSO4��7H2O)������ȱ����ƶѪҩƷ����Ҫ�ɷ֡���������������м(����������������������)Ϊԭ�����������̷���һ�ַ�����
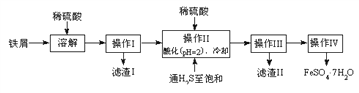
��ѯ���ϣ����й����ʵ��������±���
25��ʱ | ����H2S��Һ | SnS������ȫ | FeS��ʼ���� | FeS������ȫ |
pHֵ | 3.9 | 1.6 | 3.0 | 5.5 |
��1�������Ƶõ��̷��������Ƿ���Fe3+�����ѡ�õ��Լ�Ϊ____________________��
A.KSCN��ҺB.NaOH��ҺC.KMnO4��Һ
��2������II�У�ͨ�����������͵�Ŀ���ǣ�д���㣩___________��____________��
��3������IV��˳������Ϊ________����ȴ�ᾧ�����ˡ�
��4���ⶨ�̷���Ʒ��Fe2+�����ķ����ǣ�
a.��ȡ3.72g�̷���Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
�ٵζ�ʱʢ��KMnO4��Һ������Ϊ_________�����������ƣ���
�ڼ���������Ʒ��FeSO47H2O����������Ϊ__________��
�������ⶨ�У����ζ��ܹ��Ϊ50mL������a�г�ȡ��Ʒ���������ܳ���______g��(����4λС��)
����Ŀ��ijѧϰС�鰴����ʵ������̽�������е⺬���IJⶨ�͵����ȡ��
ʵ��(һ) �⺬���IJⶨ
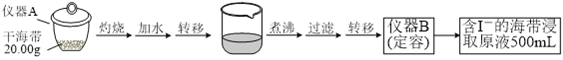
ȡ0.0100 mol��L��1��AgNO3����Һװ��ζ��ܣ�ȡ100.00 mL������ȡԭҺ���ζ��أ��õ��Ƶζ����ⶨ�⺬������õĵ綯��(E) ��ӳ��Һ��c(I��)�ı仯�������������±���
V(AgNO3)/mL | 15.00 | 19.00 | 19.80 | 19.98 | 20.00 | 20.02 | 21.00 | 23.00 | 25.00 |
E/mV | ��225 | ��200 | ��150 | ��100 | 50.0 | 175 | 275 | 300 | 325 |
ʵ��(��) �����ȡ
���ƺ�����ȡԭҺ���ס�������ʵ�鷽�����£�
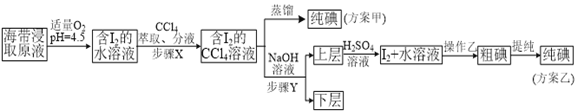
��֪��3I2��6NaOH��5NaI��NaIO3��3H2O��
��ش�
��1��ʵ��(һ) �е��������ƣ�����A_________�� ���� B___________________��
��2���ôεζ��յ�ʱ��ȥAgNO3��Һ�����Ϊ20.00mL������ú����е�İٷֺ���Ϊ_______%��
��3���ٷ�Һ©��ʹ��ǰ���©����©����Ϊ___________________��
�ڲ���X�У���ȡ���Һ©���ڹ۲쵽��������_______________��
�������йز���Y��˵������ȷ����___________________��
A.Ӧ����NaOH��Һ��Ũ�Ⱥ���� B.����ת�������ӽ���ˮ��
C.��Ҫ�dz�ȥ������ȡԭҺ�е��л����� D.NaOH��Һ�������Ҵ�����
��ʵ��(��) �в���Z��������______________________��
��4�������������������������_____________________��