��Ŀ����
����Ŀ������ʵ�ˮ��Һ�д��ڵ���ƽ�⡣
��.��1�������dz��������ᡣ���з����У�����ʹ����ϡ��Һ��CH3COOH����̶��������_____������ĸ��ţ���
a �μ�����Ũ���� b ����Һ c ��ˮϡ�� d �������������ƾ���
�״��dz����������ζ���ϣ��״�����Ȳⶨ�������¡�
a ��ȡ20.00 mL�״���Ʒ����100 mL����ƿ���Ƴɴ���Һ��
b ���ζ���ϴ������ϴ��װ����Һ���ϳ����촦���ݣ�����Һ����0�̶��ߡ�
c ȡ20.00 mL���ƵĴ���Һ�ڽྻ����ƿ�У���3�η�̪��Һ����0.1000 mol�� L��1��NaOH��Һ�ζ����յ㣬��¼���ݡ�
d �ظ��ζ�ʵ��3�β���¼���ݡ�
e ����״���Ʒ�д�������ȡ��ش��������⣺
��2��ʵ��a����ȡ20.00 mL�״����õ�����������___________��
��3����ʵ��b�м�ʽ�ζ���δ��NaOH����Һ��ϴ������ɲⶨ�����ȷֵ_________������ƫ��������ƫС����������������
��4��ʵ��C���жϵζ��յ��������_______��
��5��ʵ���������±�����ð״���Ʒ�д��������Ϊ_________ mol�� L��1��
����Һ���/mL | ��NaOH��Һ | ||
�ζ�ǰ����/mL | �ζ��յ����/mL | ||
��1�� | 20.00 | 0 | 21.98 |
��2�� | 20.00 | 0 | 22.00 |
��3�� | 20.00 | 0 | 22.02 |
��.25�棬��20.00 mL 0.100 0 mol��L1 CH3COOH�еμ�0.100 0 mol��L��1 NaOH�����У�pH�仯����ͼ��ʾ��
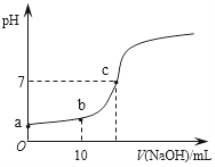
��6��a����ҺpH �� 1���õ��뷽��ʽ����ԭ��_____________��
��7�������й�b����Һ��˵����ȷ����_______������ĸ��ţ���
a ������CH3COOH��CH3COONa
b ��Ũ�����㣺c��Na���� + c��H���� = c��CH3COO���� + c��OH��
c ��Ũ�����㣺c��Na���� = c��CH3COOH�� + c��CH3COO����
d ��Ũ�����㣺2c��H���� + c��CH3COO���� =2c��OH���� +c��CH3COOH��
��8��c����Һ������Ũ�ȵĴ�С˳��__________��
���𰸡�b��c ��ʽ�ζ��� ƫ�� ��ƿ����Һ��ɫ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ 0.5500 CH3COOH ![]() CH3COO- + H+ a��b c��CH3COO-��=c��Na+��> c��H+��= c��OH-��
CH3COO- + H+ a��b c��CH3COO-��=c��Na+��> c��H+��= c��OH-��
��������
��1��a.�μ�����Ũ���ᣬ������Ũ���������ƴ���ĵ��룻
b.����Һ��Խ��Խ���룬�ٽ�����ĵ��룻
c.��ˮϡ�ͣ�ԽϡԽ���룬�ٽ�����ĵ��룻
d.�������������ƾ��壬�����Ũ������ ���ƴ���ĵ��룻
��2���ζ��ܿ��Ծ�ȷ��0.01mL���Ұ״������ԣ�
��3����ʽ�ζ���δ��NaOH����Һ��ϴ����ɱ�Һ��ϡ�ͣ����ı�Һ���ƫ��
��4�����������Ƶζ����ᣬ�ҵμ��˷�̪��ָʾ�����ʵζ��յ����������ƿ����Һ��ɫ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
��5������C1V1=C2V2��![]() �����C�����״���Ʒϡ��5���õ�����Һ��
�����C�����״���Ʒϡ��5���õ�����Һ��
��6����Ϊ����Ϊ���ᣬ����0.1mol/L�Ĵ���pH>1��
��7��b�������������10mL�������ᷴӦһ�룬ʣ��һ�룬��b������Ϊ����ʹ�����1:1��ϣ�
��8��c�������ԣ���c��H+��= c��OH-�������ݵ���غ㣬c��CH3COO-��=c��Na+����
��1��a.�μ�����Ũ���ᣬ������Ũ���������ƴ���ĵ��룬a����
b.����Һ��Խ��Խ���룬�ٽ�����ĵ��룬b��ȷ��
c.��ˮϡ�ͣ�ԽϡԽ���룬�ٽ�����ĵ��룬c��ȷ��
d.�������������ƾ��壬�����Ũ������ ���ƴ���ĵ��룬d����
��ѡbc��
��2����ȡ20.00 mL�״ף��ζ��ܿ��Ծ�ȷ��0.01mL���Ұ״������ԣ���ѡ����ʽ�ζ��ܣ�
��Ϊ����ʽ�ζ��ܣ�
��3����ʽ�ζ���δ��NaOH����Һ��ϴ����ɱ�Һ��ϡ�ͣ����ı�Һ���ƫ�� �ʲ�ô���ҺŨ��ƫ��
�ʴ�Ϊ��ƫ��
��4�����������Ƶζ����ᣬ�ҵμ��˷�̪��ָʾ�����ʵζ��յ����������ƿ����Һ��ɫ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
��Ϊ����ƿ����Һ��ɫ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
��5�����ݱ������ݣ�����ʵ�����ı�Һ��ƽ�����Ϊ22.00mL������C1V1=C2V2��![]() �����C��=0.1100mol/L���״���Ʒϡ��5���õ�����Һ���ʰ״���Ʒ��Ũ��Ϊ
�����C��=0.1100mol/L���״���Ʒϡ��5���õ�����Һ���ʰ״���Ʒ��Ũ��Ϊ![]() ��
��
�ʴ�Ϊ��0.5500��
��6����Ϊ����Ϊ���ᣬ����0.1mol/L�Ĵ���pH>1��![]() ��
��
�ʴ�Ϊ��![]() ��
��
��7��b�������������10mL�������ᷴӦһ�룬ʣ��һ�룬��b������Ϊ����ʹ�����1:1��ϣ�
a.����Ϊ����ʹ����ƣ�a��ȷ��
b.����غ�:![]() ,b��ȷ��
,b��ȷ��
c.�����غ㣺![]() ��c����
��c����
d.���ݵ���غ�������غ���ӵ������غ㣺![]() ��d����
��d����
��ѡab��
��8��c�������ԣ���c��H+��= c��OH-�������ݵ���غ㣬c��CH3COO-��=c��Na+���������Ӻ�������Ũ�Ⱥ�С����![]() ��
��
����![]() ��
��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�����Ŀ��ijͬѧ��NaOH��Һ���ζ��ڱ������������Һ�����в�����
������Һ�м���1��2��ָʾ��
��ȡ20.00 mL����Һ������ƿ��
����NaOH��Һ�ζ����յ㣨�յ�ʱ��Һ��pHԼΪ9.1��
���ظ����ϲ���
������ƽ��ȷ��ȡһ�������ڱ���������ع������250 mL����Һ�����pHԼΪ4.2��
����ʵ�����ݼ���NaOH�����ʵ���Ũ��
��1�����ϸ����У���ȷ�ģ�����ţ�����˳����_____________________________����������ʹ�õ���������ƿ�⣬����Ҫʹ�õ�������______________��
��2��ѡ��ָʾ����______________���ζ��յ�����______________________________________��
��3���ζ�������¼NaOH���ն������ظ��ζ����Σ����ݼ�¼���±���
�ζ�����ʵ������ | 1 | 2 | 3 | 4 |
V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
V��NaOH��/mL���������� | 0.10 | 0.30 | 0.00 | 0.20 |
V��NaOH��/mL���ն����� | 20.08 | 20.30 | 20.80 | 20.22 |
V��NaOH��/mL�����ģ� | 19.98 | 20.00 | 20.80 | 20.02 |
ijͬѧ�ڴ������ݹ����м���õ�ƽ������NaOH��Һ�����ΪV��NaOH������19.98+20.00+20.80+20.02��/4mL��20.20mL�����ļ��������������_______________��
��4����������ڹ۲�ζ��ܵ���ʼ����ʱ��Ҫʹ�ζ��ܵļ��첿�ֳ�����Һ���ζ�ǰ������ˮϴ����ʽ�ζ��ܣ�Ȼ���NaOH��Һ���еζ����˲�����ʵ����________������ƫ��������ƫС��������Ӱ��������