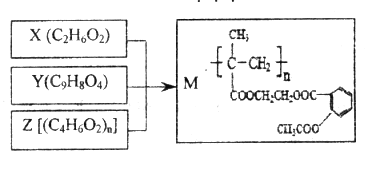
ЬтФПФкШн
ЁОЬтФПЁПЫсЁЂМюЁЂбЮЖМЪєгкЕчНтжЪЃЌЪЧЙЄвЕЩњВњЁЂПЦбаСьгђЕФживЊдСЯЃЌЧыАДвЊЧѓЛиД№ЯТСаЮЪЬтЃК
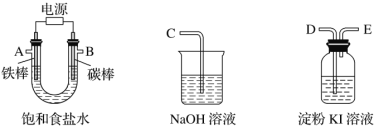
(1)ЯжгаЫФжжЯЁШмвКЃЌЗжБ№ЪЧЃКA.СђЫсШмвК B.МзЫсШмвК C. NaOHШмвК D.АБЫЎ
ШЁЦфжаСНжжШмвКЗЂЩњжаКЭЗДгІЃЌЧыбЁдёКЯЪЪЕФЖдЯѓЭъГЩЬтФПЕФНтД№ЃК
ЂйAКЭCЕФЯЁШмвКЗЂЩњжаКЭЗДгІЩњГЩ1molЫЎЪБЃЌЗХГі57.3kJЕФШШСПЃЌЧыаДГіДЫжаКЭЗДгІЕФШШЛЏбЇЗНГЬЪН______________________________.
ЂкЫсМюжаКЭЕЮЖЈЪБЃЌаыбЁдёКЯЪЪЕФжИЪОМСЃЌЯТСаЫсМюзщКЯНјааЕЮЖЈЪБЃЌВЛФмбЁдёМзЛљГШЮЊжИЪОМСЪЧ____(бЁЬюзжФИ)
a.AКЭC b.AКЭD c. BКЭ C d. BКЭD
ЂлBжаМзЫсЕФгУЭОжЎвЛЪЧгУгкХфжЦЁАЛКГхШмвКЁБ(HCOOH~HCOONa)ЁЃЧыаДГіМзЫсЕчРыЗНГЬЪН___________________________ЃЛвбжЊвЛЖЈЮТЖШЯТЃЌМзЫсЕФЕчРыЦНКтГЃЪ§ Ka=1.8ЁС10Ѓ4ЃЌШєгУ 0.2molЁЄLЃ1HCOOH ШмвК100mLХфжЦpHЮЊ4ЕФЛКГхШмвКЃЌашМгШы_______mL(Д№АИБЃСєвЛЮЛаЁЪ§) 0.2molЁЄ LЃ1NaOHШмвКЁЃ
(2)Щщ(As)ЪЧвЛаЉЙЄГЇКЭПѓЩНЗЯЫЎжаЕФЮлШОдЊЫиЃЌH3AsO3(бЧЩщЫс)КЭH3AsO4(бЧЩщЫс)ЫЎШмвКжаКЌЩщЕФИїЮяжжЕФЗжВМЗжЪ§(ЦНКтЪБФГЮяжжЕФХЈЖШеМИїЮяжжХЈЖШзмКЭЕФБШжЕ)гыpHЙиЯЕЗжБ№ШчЭМЫљЃК
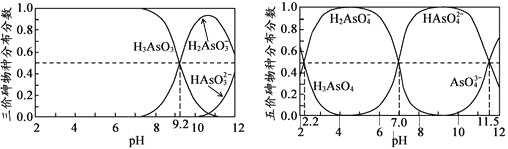
ЂйвдЗгЬЊЮЊжИЪОМСЃЌНЋNaOHШмвКж№ЕЮМгШыЕНH3AsO3ШмвКжа,ЕБШмвКгЩЮоЩЋБфЮЊЧГКьЩЋЪБЭЃжЙЕЮМгЃЌДЫЪБЩщдЊЫиЕФжївЊДцдкаЮЪНЮЊ________________(ЬюЮЂСЃЕФЛЏбЇЪН)ЂквбжЊ pKa1 = ЁЊlgKa1
H3AsO4ЕквЛВНЕчРыЗНГЬЪНЮЊH3AsO4![]() H2AsO4- + H+ ЕчРыГЃЪ§ЮЊKa1,дђpKa1=______ЁЃ
H2AsO4- + H+ ЕчРыГЃЪ§ЮЊKa1,дђpKa1=______ЁЃ
ЁОД№АИЁП![]() H2SO4(aq)+NaOH(aq)=
H2SO4(aq)+NaOH(aq)=![]() Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/mol c HCOOH
Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/mol c HCOOH![]() HCOO-+ H+ 64.3 H2AsO3-ЁЂH3AsO3 2.2
HCOO-+ H+ 64.3 H2AsO3-ЁЂH3AsO3 2.2
ЁОНтЮіЁП
ЃЈ1ЃЉЂйЧПЫсКЭЧПМюдкЯЁШмвКжаЗЂЩњжаКЭЗДгІЩњГЩ1molЫЎЪБЗХГіЕФШШСПЮЊжаКЭШШЃЌОнДЫаДГіжаКЭШШЕФШШЛЏбЇЗНГЬЪНЃЛ
ЂкШѕЫсгыЧПМюЗДгІЩњГЩЧПМюШѕЫсбЮЃЌШмвКЯдМюадЃЌгІбЁдёМюаджИЪОМСЃЛ
ЂлМзЫсЮЊШѕЫсЃЌЕчРыЗНГЬЪНЮЊHCOOH![]() HCOO-+ H+ЃЛШєгУ100mL 0.2molL-1HCOOHШмвКХфжЦpHЮЊ4ЕФЛКГхШмвКЃЌдђ
HCOO-+ H+ЃЛШєгУ100mL 0.2molL-1HCOOHШмвКХфжЦpHЮЊ4ЕФЛКГхШмвКЃЌдђ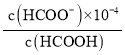 =1.8ЁС10-4ЃЌПЩжЊc(HCOO-)=1.8c(HCOOH)ЃЌдШмвКжаn(HCOOH)=0.02molЃЌЩшМгШыxmolNaOHЃЌдђx=1.8(0.02-x)ЃЌx=0.01286molЃЌОнДЫМЦЫуГіNaOHШмвКЕФЬхЛ§ЃЛ
=1.8ЁС10-4ЃЌПЩжЊc(HCOO-)=1.8c(HCOOH)ЃЌдШмвКжаn(HCOOH)=0.02molЃЌЩшМгШыxmolNaOHЃЌдђx=1.8(0.02-x)ЃЌx=0.01286molЃЌОнДЫМЦЫуГіNaOHШмвКЕФЬхЛ§ЃЛ
(2)ЂйИљОнЭМжЊЃЌМюаддіЧПЪБЃЌH3AsO3ЕФХЈЖШМѕаЁЁЂH2AsO3-ХЈЖШдіДѓЃЌЫЕУїМюКЭH3AsO3ЩњГЩH2AsO3-ЁЃЗгЬЊЕФБфЩЋЗЖЮЇЪЧ8.2-10.0ЃЌЕБШмвКЕФPHдк8.2ЕН10.0жЎМфЪБЃЌШмвКЯдЗлКьЩЋЃЌОнДЫЗжЮіЩщдЊЫиЕФжївЊДцдкаЮЪНЃЛ
ЂкKa1=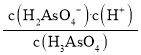 ЃЌpH=2.2ЪБc(H+)=10-2.2 mol/LЃЌc(H3AsO3)=c(H2AsO3-)ЃЌОнДЫМЦЫуГіKa1ЃЌИљОнpKa1=-lgKa1ПЩМЦЫуГіНсЙћЁЃ
ЃЌpH=2.2ЪБc(H+)=10-2.2 mol/LЃЌc(H3AsO3)=c(H2AsO3-)ЃЌОнДЫМЦЫуГіKa1ЃЌИљОнpKa1=-lgKa1ПЩМЦЫуГіНсЙћЁЃ
(1)ЂйЧПЫсКЭЧПМюдкЯЁШмвКжаЗЂЩњжаКЭЗДгІЩњГЩ1molЫЎЪБЗХГі57.3kJЕФШШСПЃЌЙЪбЁдёСђЫсгыЧтбѕЛЏФЦЃЌДЫжаКЭЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ![]() H2SO4(aq)+NaOH(aq)=
H2SO4(aq)+NaOH(aq)=![]() Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/molЃЛ
Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/molЃЛ
ЙЪД№АИЮЊЃК![]() H2SO4(aq)+NaOH(aq)=
H2SO4(aq)+NaOH(aq)=![]() Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/molЃЛ
Na2SO4(aq)+H2O(l) ЁїH=-57.3kJ/molЃЛ
ЂкШѕЫсгыЧПМюЗДгІЩњГЩЧПМюШѕЫсбЮЃЌШмвКЯдМюадЃЌВЛФмбЁдёМзЛљГШЮЊжИЪОМСЃЌЙЪД№АИЮЊЃКcЃЛ
ЂлМзЫсЮЊШѕЫсЃЌЕчРыЗНГЬЪНЮЊHCOOH![]() HCOO-+ H+ЃЛШєгУ100mL 0.2molL-1HCOOHШмвКХфжЦpHЮЊ4ЕФЛКГхШмвКЃЌдђ
HCOO-+ H+ЃЛШєгУ100mL 0.2molL-1HCOOHШмвКХфжЦpHЮЊ4ЕФЛКГхШмвКЃЌдђ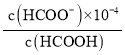 =1.8ЁС10-4ЃЌПЩжЊc(HCOO-)=1.8c(HCOOH)ЃЌдШмвКжаn(HCOOH)=0.02molЃЌЩшМгШыxmolNaOHЃЌдђx=1.8(0.02-x)ЃЌx=0.01286molЃЌдђашМгШы0.2molL-1NaOHШмвКЕФЬхЛ§ЮЊ
=1.8ЁС10-4ЃЌПЩжЊc(HCOO-)=1.8c(HCOOH)ЃЌдШмвКжаn(HCOOH)=0.02molЃЌЩшМгШыxmolNaOHЃЌдђx=1.8(0.02-x)ЃЌx=0.01286molЃЌдђашМгШы0.2molL-1NaOHШмвКЕФЬхЛ§ЮЊ![]() =0.0643L=64.3mLЃЛ
=0.0643L=64.3mLЃЛ
ЙЪД№АИЮЊЃКHCOOH![]() HCOO-+ H+ЃЛ64.3ЃЛ
HCOO-+ H+ЃЛ64.3ЃЛ
(2)ЂйИљОнЭМжЊЃЌМюаддіЧПЪБЃЌH3AsO3ЕФХЈЖШМѕаЁЁЂH2AsO3-ХЈЖШдіДѓЃЌЫЕУїМюКЭH3AsO3ЩњГЩH2AsO3-ЁЃЗгЬЊЕФБфЩЋЗЖЮЇЪЧ8.2-10.0ЃЌЕБШмвКЕФPHдк8.2ЕН10.0жЎМфЪБЃЌШмвКЯдЗлКьЩЋЃЌДЫЪБЩщдЊЫиЕФжївЊДцдкаЮЪНЮЊH2AsO3-ЁЂH3AsO3ЃЛ
ЙЪД№АИЮЊЃКH2AsO3-ЁЂH3AsO3ЃЛ
ЂкKa1=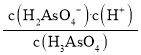 ЃЌpH=2.2ЪБc(H+)=10-2.2 mol/LЃЌc(H3AsO3)=c(H2AsO3-)ЃЌpKa1=-lgKa1=-lg
ЃЌpH=2.2ЪБc(H+)=10-2.2 mol/LЃЌc(H3AsO3)=c(H2AsO3-)ЃЌpKa1=-lgKa1=-lg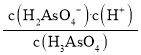 =2.2ЁЃ
=2.2ЁЃ
ЙЪД№АИЮЊЃК2.2ЁЃ

 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ