��Ŀ����
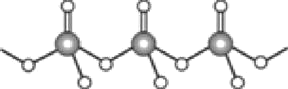
����Ŀ����֪��2RCH2CHO![]() RCH2CH=CRCHO��H2O��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
RCH2CH=CRCHO��H2O��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�

��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ_________���ṹ������ʾAֻ��һ������A������Ϊ___________��
��2��B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________________��
��3��C��____�ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���______________________________________________________��
��4�������ķ�Ӧ����Ϊ_________________��D���������ŵ�����Ϊ________________��
��5��д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��_________________________��
a�������к���6��̼ԭ����һ������ b�����������������Ű���ˮ������еĹ�����
��6���������ķ�Ӧ����Ϊ___________��д��E�Ľṹ��ʽ_________________________��
���𰸡�C4H1OO 1-���������������� CH3CH2CH2CHO+2Cu(OH)2+NaOH![]() CH3CH2CH2COONa+Cu2O��+3H2O 2 ������Һ��ϡ���ᡢ��ˮ�������������𰸣� ��ԭ��Ӧ����ӳɷ�Ӧ�� �ǻ� HOCH2C��C��C��CCH2COOH��HOCH2CH2C��C��C��C��COOH��CH3C��C��C��CCH (OH)COOH��CH3CH (OH)C��C��C��C��COOH Ũ���ᡢ����
CH3CH2CH2COONa+Cu2O��+3H2O 2 ������Һ��ϡ���ᡢ��ˮ�������������𰸣� ��ԭ��Ӧ����ӳɷ�Ӧ�� �ǻ� HOCH2C��C��C��CCH2COOH��HOCH2CH2C��C��C��C��COOH��CH3C��C��C��CCH (OH)COOH��CH3CH (OH)C��C��C��C��COOH Ũ���ᡢ���� 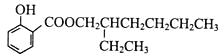
��������
����һԪ�����������������������ʽΪC4H10O������Է�������Ϊ74��A������������B��Ȼ��B������������Ϣ�ķ�Ӧ����C����C����Է�������Ϊ126��D����Է�������Ϊ130������C��D�����˴����ⷴӦ��D��ˮ���ᷴӦ����E��
(1)AΪһԪ��������������������ԼΪ21.6%��������Է�������Mr��16��21.6%��74�������ʽΪCxHyO����12x��y��16��74��12x��y��58����x��4ʱ��y��10������ʽΪC4H10O����x��3��x��5ʱ��������������A�ķ���ʽΪC4H10O��A��ֻ��һ����������AΪCH3CH2CH2CH2OH������Ϊ1������(��������)��
(2)����������֪BΪCH3CH2CH2CHO��B�����Ƶ�Cu(OH)2�ķ�ӦΪCH3CH2CH2CHO��2Cu(OH)2��NaOH![]() CH3CH2CH2COONa��Cu2O����3H2O��
CH3CH2CH2COONa��Cu2O����3H2O��
(3)������ṩ����Ϣ��֪������C������2�ֽṹ���ֱ�Ϊ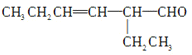 ��
��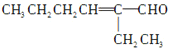 ����CHO��
����CHO��![]() ��ͬ����ʱ��Ҫ�ȼ��飭CHO��Ȼ�����
��ͬ����ʱ��Ҫ�ȼ��飭CHO��Ȼ�����![]() �������Լ�Ϊ������Һ��ϡ�������ˮ��(4)C����Է�������Ϊ126��D����Է�������Ϊ130������C��D����H2�����ӳɷ�Ӧ��Ҳ��Ϊ��ԭ��Ӧ��������Է����������4������
�������Լ�Ϊ������Һ��ϡ�������ˮ��(4)C����Է�������Ϊ126��D����Է�������Ϊ130������C��D����H2�����ӳɷ�Ӧ��Ҳ��Ϊ��ԭ��Ӧ��������Է����������4������![]() �ͣ�CHO����H2�ӳɣ�����D�й���������Ϊ�ǻ���
�ͣ�CHO����H2�ӳɣ�����D�й���������Ϊ�ǻ���
(5)ˮ����IJ����Ͷ�Ϊ5����ͬ���칹������к�����һ�����ϵ�6��̼ԭ�ӣ� ���������Ű���ˮ������еĹ����ţ��������ǻ����ǻ����ʿ��ܵĽṹ��HOCH2C��C��C��CCH2COOH��HOCH2CH2C��C��C��C��COOH��CH3C��C��C��CCH (OH)COOH��CH3CH (OH)C��C��C��C��COOH��4�֣�
(6)������Ϊ������Ӧ������������ŨH2SO4�����ȣ�E�Ľṹ��ʽΪ ��
��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�����Ŀ����1�������г�����һЩԭ�ӵ�2p�ܼ���3d�ܼ��е����Ų�����������ж���ЩΥ��������ԭ��________����ЩΥ���˺��ع���________��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��2��![]() ����������������ӽṹ��ͼ��ʾ��
����������������ӽṹ��ͼ��ʾ��

![]() ��������ԭ�ӵ��ӻ��������Ϊ________��
��������ԭ�ӵ��ӻ��������Ϊ________��
![]() ÿ��
ÿ��![]() �����к��еŵ��ӶԵ���ĿΪ________��
�����к��еŵ��ӶԵ���ĿΪ________��
��3����ѧ�Һϳ���һ����������![]() ������ṹ�ǶԳƵģ�5��N�ų���V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2�������������˺��ֺϳ���һ�ֺ�����
������ṹ�ǶԳƵģ�5��N�ų���V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2�������������˺��ֺϳ���һ�ֺ�����![]() ���Ļ�ѧʽΪ��
���Ļ�ѧʽΪ��![]() �������Ӿ��壬�����ʽΪ________������
�������Ӿ��壬�����ʽΪ________������![]() �м����֮��ļн�Ϊ
�м����֮��ļн�Ϊ![]() �����жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ṹʽΪ________________��
�����жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ṹʽΪ________________��
��4��ֱ�������������������������������������������ͨ�����ö�����ԭ�����������ģ���ͼ��ʾ������n�������������γɵ�������������ӵ�ͨʽΪ________��
��5��̼�����е������Ӳ�ͬ���ȷֽ��¶ȾͲ�ͬ���±�Ϊ����̼���ε��ȷֽ��¶Ⱥͽ��������Ӱ뾶
̼���� |
|
|
|
|
�ȷֽ��¶� | 402 | 900 | 1172 | 1360 |
���������Ӱ뾶 | 66 | 99 | 112 | 135 |
���Ž��������Ӱ뾶������̼���ε��ȷֽ��¶������ߣ�ԭ����_____________��
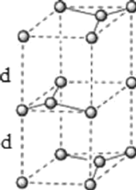
��6��ʯī�ľ���ṹ�;����ṹ��ͼ��ʾ����֪ʯī���ܶ�Ϊ![]() ��
��![]() ���ļ���Ϊ
���ļ���Ϊ![]() �������ӵ�������ֵΪ
�������ӵ�������ֵΪ![]() ����ʯī����IJ���Ϊ________cm��
����ʯī����IJ���Ϊ________cm��
 ��
��
����Ŀ���������Ϊ5 mL�ļס��ҡ�������Һ���������Թܱڻ��������Թ���(����)��������ͼ��ʾ��ʵ��������ס��ҡ�������Ͽ�����(����)
![]()
ѡ�� | A | B | C | D |
�� | 1,2�������� | �屽 | ˮ | �Ҵ� |
�� | ˮ | Һ�� | ���� | ���� |
�� | �� | �Ҵ� | ��ˮ | �������� |
A. A B. B C. C D. D