��Ŀ����
16��������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ������ش��������⣺��1����̬Siԭ���У�����ռ�ݵ�����ܲ����ΪM�����ܲ���е�ԭ�ӹ����Ϊ9��������Ϊ4��
��2������Ҫ�Թ����Ρ���������Ȼ��������ʽ�����ڵؿ��У�
��3�����ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮���Թ��ۼ����ϣ��侧���й���8��ԭ�ӣ�����������λ�ù���3��ԭ�ӣ�
��4�����ʹ��ͨ�����飨SiH4���ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg2Si��NH4Cl��Һ�������з�Ӧ�Ƶ�SiH4���÷�Ӧ�Ļ�ѧ����ʽΪMg2Si+4NH4Cl=SiH4+4NH3+2MgCl2��
��5��̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
| ��ѧ�� | C-C | C-H | CһO | Si-Si | Si-H | SiһO |
| ����/��kJ•mol-1�� | 356 | 413 | 336 | 226 | 318 | 452 |
��SiH4���ȶ���С��CH4���������������ԭ����C-H���ļ��ܴ���C-O����C-H����C-O���ȶ�����Si-H���ļ���ȴԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O����
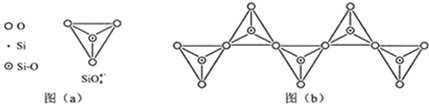
��6���ڹ������У�SiO44-�����壨��ͼ��a����ͨ�����ö��������ӿ��γɵ�״����״����״���Ǽ���״�Ĵ���ṹ��ʽ��ͼ��b��Ϊһ�����������ṹ�Ķ�����������Siԭ�ӵ��ӻ���ʽΪsp3��Si��O��ԭ����֮��Ϊ1��3��
���� ��1��Siԭ�Ӻ��������Ϊ14����̬ԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p2���ݴ˽��
��2��������Ȼ������Ҫ�Թ����κͶ���������ʽ���ڣ�
��3���辧��ͽ��ʯ�������ƶ�����ԭ�Ӿ��壬��ԭ��֮���Թ��ۼ���ϣ��ڽ��ʯ����ľ����У�ÿ��������һ��̼ԭ�ӣ���������ƽṹ����������λ�ù���ԭ��Ϊ 6��$\frac{1}{2}$=3����
��4��Mg2Si��NH4Cl��Һ�������з�Ӧ�Ƶ�SiH4��NH3��MgCl2��
��5���ټ���ԽС����ѧ��Խ���ȶ���
�ڼ���ԽС����ѧ��Խ���ȶ�����Ӧ�������γ��ȶ��Ը�ǿ������У�
��6���������еĹ������SiO44-��Ϊ��������ṹ����������ԭ��Siԭ�Ӳ�ȡ��sp3�ӻ���ʽ������ͼ��b����һ���ṹ��Ԫ�к���1���衢3����ԭ�ӣ�
��� �⣺��1��Siԭ�Ӻ��������Ϊ14����̬ԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p2������ռ�ݵ�����ܲ����ΪM�����ܲ���е�ԭ�ӹ����Ϊ1+3+5=9��������Ϊ4��
�ʴ�Ϊ��M��9��4��
��2��������Ȼ������Ҫ�Թ����κͶ���������ʽ���ڣ�
�ʴ�Ϊ���������裻
��3���辧��ͽ��ʯ�������ƶ�����ԭ�Ӿ��壬��ԭ��֮���Թ��ۼ���ϣ��ڽ��ʯ����ľ����У�ÿ��������һ��̼ԭ�ӣ���������ƽṹ����������λ�ù���ԭ��Ϊ 6��$\frac{1}{2}$=3����
�ʴ�Ϊ��3��
��4��Mg2Si��NH4Cl��Һ�������з�Ӧ�Ƶ�SiH4��NH3��MgCl2����Ӧ�Ļ�ѧ����ʽΪ��Mg2Si+4NH4Cl=SiH4+4NH3+2MgCl2��
�ʴ�Ϊ��Mg2Si+4NH4Cl=SiH4+4NH3+2MgCl2��
��5�����ɱ������ݿ�֪��C-C����C-H����ǿ�����γɵ������ȶ�����������Si-Si����Si-H���ļ��ܽϵͣ����ѣ����³��������������ɣ�
�ʴ�Ϊ��C-C����C-H����ǿ�����γɵ������ȶ�����������Si-Si����Si-H���ļ��ܽϵͣ����ѣ����³��������������ɣ�
���ɱ������ݿ�֪��C-H���ļ��ܴ���C-O����C-H����C-O���ȶ�����Si-H���ļ���ȴԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O����
�ʴ�Ϊ��C-H���ļ��ܴ���C-O����C-H����C-O���ȶ�����Si-H���ļ���ȴԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O����
��6���������еĹ������SiO44-��Ϊ��������ṹ����������ԭ��Siԭ�Ӳ�ȡ��sp3�ӻ���ʽ��
����ͼ��b����һ���ṹ��Ԫ�к���1���衢3����ԭ�ӣ�
�ʴ�Ϊ��sp3��1��3��
���� ������Ҫ�����˻�̬ԭ�ӵĺ�������Ų�������ṹ����ѧ����ʽ����д���ӻ�������Ѷ��еȣ�����ʱע����ܶ��������ʼ��ṹ��Ӱ�죮

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�| ʵ����������� | ����Ľ��� | |
| A | ��һƬ�������ھƾ������������գ������ۻ��������� | ���������۵��ر�� |
| B | �ò������쵼�ܵ�����������ȼ���۲쵽����ʻ�ɫ | ��ͨ�����к�����Ԫ�� |
| C | ��ˮ�м���Na2O2���壬�����̪�ʺ�ɫ������ɫ��ȥ | �����˼������ʣ���Na2O2����Ư���� |
| D | ������ı���Ũ��Һ�еμ�����������ˮ����δ�۲쵽��ɫ�������� | ���屽���ܽ��ڹ����ı����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���·������������ۻ�Ϊͬ���칹�� | |
| B�� | ��ըʳ��Ļ����ͺ�ţ�Ͷ��ǿ������ı������� | |
| C�� | ĥ�����Ĵ��������ʣ�������к��ʱ���˰����� | |
| D�� | ʩ��ʱ����ľ�ң���Ч�ɷ�ΪK2CO3����NH4Cl���ʹ�ã�������������ͷ�Ч |
| A�� | ����������Һ�еμӹ����İ�ˮ��Al3++4OH-�TAlO2-+2H2O | |
| B�� | ����ͭ��Ũ���ᷴӦ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O | |
| C�� | ʵ�����Ʊ������������壺Fe3++3H2O�TFe��OH��3�����壩+3H+ | |
| D�� | ������Һ��ͨ�������Ķ�����̼���壺2C6H5ONa+CO2+H2O�T2C6H6O+Na2CO3 |
| ѡ�� | ���� | ���� |
| A | �ǽ�����Խǿ������̬�⻯��Խ�ȶ� | �ȶ��ԣ�NH3��PH3 |
| B | ��Ӧ��Ũ��Խ��Ӧ����Խ�� | �����£���ͬ����Ƭ�зֱ����������Ũ��ϡ���ᣬŨ��������Ƭ���ܽ��� |
| C | �ṹ��������Ƶ����ʣ��е�����Է���������������� | HF�е����HCl |
| D | Ũ����ܸ�����л�ԭ�Ե����� | Ũ������Ը���SO2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ��
��
 ��
��