��Ŀ����
��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X���л�����Ҫ���Ԫ�ء�X��һ��1��1����̬�⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ��,Z�ĺ˵����С��28,�Ҵ������2��δ�ɶԵ��ӡ�
(1)X�ڸ��⻯����������������ʽ�ӻ���X��Y�γɵĻ�������۵�Ӧ����������(����ڡ����ڡ�)X�⻯����۵㡣
(2)Y�����ڱ���λ��������������������������;Z4+�ĺ�������Ų�ʽΪ����������
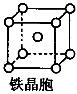
(3)��ҵ������ZO2��̼�ᱵ������״̬����ȡ������M(M�ɿ���һ�ֺ�������)����X���߷���,M�������С�ظ���λΪ������(��ͼ),�߳�Ϊ4.03��10-10 m,����λ��ΪZ4+��ռ,����λ��ΪBa2+��ռ,��������λ��ΪO2-��ռ��
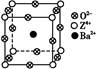
���Ʊ�M�Ļ�ѧ��Ӧ����ʽ��������������������������(Z��Ԫ�ط��ű�ʾ)��
����M������,Ba2+������λ��(Ba2+��Χ�Ⱦ��������O2-����Ŀ)Ϊ����������
�۾���M�ܶȵļ���ʽΪ��=��������������������(Z���ԭ������Ϊ48)��
(1)X�ڸ��⻯����������������ʽ�ӻ���X��Y�γɵĻ�������۵�Ӧ����������(����ڡ����ڡ�)X�⻯����۵㡣
(2)Y�����ڱ���λ��������������������������;Z4+�ĺ�������Ų�ʽΪ����������
(3)��ҵ������ZO2��̼�ᱵ������״̬����ȡ������M(M�ɿ���һ�ֺ�������)����X���߷���,M�������С�ظ���λΪ������(��ͼ),�߳�Ϊ4.03��10-10 m,����λ��ΪZ4+��ռ,����λ��ΪBa2+��ռ,��������λ��ΪO2-��ռ��

���Ʊ�M�Ļ�ѧ��Ӧ����ʽ��������������������������(Z��Ԫ�ط��ű�ʾ)��
����M������,Ba2+������λ��(Ba2+��Χ�Ⱦ��������O2-����Ŀ)Ϊ����������
�۾���M�ܶȵļ���ʽΪ��=��������������������(Z���ԭ������Ϊ48)��
(1)sp�����ڡ�(2)��4���ڢ�A�塡1s22s22p63s23p6
(3)��TiO2+BaCO3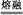 BaTiO3+CO2������12
BaTiO3+CO2������12
��(137+48+3��16)/NA(4.03��10-8)3 g��cm-3
(3)��TiO2+BaCO3
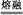 BaTiO3+CO2������12
BaTiO3+CO2������12��(137+48+3��16)/NA(4.03��10-8)3 g��cm-3
�л������Ҫ���Ԫ��XΪ̼Ԫ��,ZԪ�ش������2��δ�ɶԵ����Һ˵����С��28,ֻ��Ϊ����Ԫ����22Ti,λ�����ڱ��е�4���ڢ�B�塣����ԭ������֮��Ϊ48,����֪YΪ20��Ԫ�ظơ�(1)X�ĸ��⻯��Ϊ��Ȳ,��ȲΪֱ���η���,̼ԭ����sp��ʽ�ӻ�,̼���������ӻ�����,�۵����̼���⻯����Ӿ����۵㡣(2)��Ԫ�������ڱ��е�4���ڢ�A�塣�����Ӻ�������Ų�ʽΪ:1s22s22p63s23p6��(3)���þ�̯�������Ƴ�M�Ļ�ѧʽΪBaTiO3,�Ʊ�BaTiO3��ѧ����ʽ:TiO2+BaCO3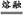 BaTiO3+CO2�������뱵���ӽ��ڵȾ�������ӹ���12��������1 mol�û�����������ϵ��:
BaTiO3+CO2�������뱵���ӽ��ڵȾ�������ӹ���12��������1 mol�û�����������ϵ��: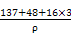 =(4.03��10-8)3NA,�����æ�=
=(4.03��10-8)3NA,�����æ�=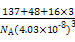 g��cm-3��
g��cm-3��
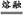 BaTiO3+CO2�������뱵���ӽ��ڵȾ�������ӹ���12��������1 mol�û�����������ϵ��:
BaTiO3+CO2�������뱵���ӽ��ڵȾ�������ӹ���12��������1 mol�û�����������ϵ��: =(4.03��10-8)3NA,�����æ�=
=(4.03��10-8)3NA,�����æ�=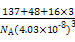 g��cm-3��
g��cm-3��
��ϰ��ϵ�д�
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
�����Ŀ
 3Na2O��2Fe��9N2����
3Na2O��2Fe��9N2����
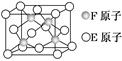

 CH��C��N���Ʊ����ڵ�ԭ��,������ЦҼ��ͦм��ĸ���֮��Ϊ��������������������ȣ�,д���÷���������̼ԭ�ӵ��ӻ���ʽ����������
CH��C��N���Ʊ����ڵ�ԭ��,������ЦҼ��ͦм��ĸ���֮��Ϊ��������������������ȣ�,д���÷���������̼ԭ�ӵ��ӻ���ʽ����������  Ϊ̼ԭ��,
Ϊ̼ԭ��, Ϊ��ԭ�ӣ���ÿ��̼ԭ����Χ�����������Ĺ�ԭ�����������������辧���߳�Ϊa cm,�ܶ�Ϊb g/cm3,���ӵ������ɱ�ʾΪ�����ú�a��b��ʽ�ӱ�ʾ����
Ϊ��ԭ�ӣ���ÿ��̼ԭ����Χ�����������Ĺ�ԭ�����������������辧���߳�Ϊa cm,�ܶ�Ϊb g/cm3,���ӵ������ɱ�ʾΪ�����ú�a��b��ʽ�ӱ�ʾ���� 


 ����1����
����1���� ����C6H6�ǷǼ��Է���
����C6H6�ǷǼ��Է���
