��Ŀ����
�������ƣ�NaN3����һ����ɫ���壬�����������Ʊ�����Ϊ2NaNH2��N2O��NaN3��NaOH��NH3��3NaNH2��NaNO3��NaN3��3NaOH��NH3����
�ش��������⣺
��1�������ڵ������У��縺������Ԫ����________����һ��������С��Ԫ����_______��
��2����̬��ԭ�ӵ�L������Ų�ͼΪ_______________��
��3����N3����Ϊ�ȵ�����ķ���Ϊ__________��д��һ�֣������ݼ۲���ӶԻ������ۣ�NO3���Ŀռ乹��Ϊ_____________��
��4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ_________________���������Ƶ�ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��_____________________________��
��5��N2O�е㣨��88��49�棩��NH3�е㣨��33��34�棩�ͣ�����Ҫԭ����__________________��
��6����ȫ���ҵ����ԭ��Ϊ6NaN3��Fe2O3 3Na2O��2Fe��9N2����
3Na2O��2Fe��9N2����
�ٵ������ЦҼ��ͦм���Ŀ֮��Ϊ________________________��
���������д��ڵĻ�ѧ������Ϊ__________________________��
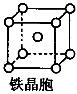
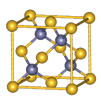
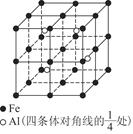
��������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������

�ش��������⣺
��1�������ڵ������У��縺������Ԫ����________����һ��������С��Ԫ����_______��
��2����̬��ԭ�ӵ�L������Ų�ͼΪ_______________��
��3����N3����Ϊ�ȵ�����ķ���Ϊ__________��д��һ�֣������ݼ۲���ӶԻ������ۣ�NO3���Ŀռ乹��Ϊ_____________��
��4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ_________________���������Ƶ�ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��_____________________________��
��5��N2O�е㣨��88��49�棩��NH3�е㣨��33��34�棩�ͣ�����Ҫԭ����__________________��
��6����ȫ���ҵ����ԭ��Ϊ6NaN3��Fe2O3
 3Na2O��2Fe��9N2����
3Na2O��2Fe��9N2�����ٵ������ЦҼ��ͦм���Ŀ֮��Ϊ________________________��
���������д��ڵĻ�ѧ������Ϊ__________________________��
��������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������
��1��������F����1�֣� ﮣ���Li����1�֣�
��2��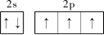 ��2�֣�
��2�֣�
��3��CO2��N2O��1�֣���ƽ�������Σ�1�֣�
��4�����Ӿ��壨1�֣���N3?+H2O HN3+OH?��2�֣�
HN3+OH?��2�֣�
��5��������֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ����2�֣�
��6��1��2��1�֣� ��������1�֣� ��112/NA��a3 g��cm-3��2�֣�����
��2��
 ��2�֣�
��2�֣���3��CO2��N2O��1�֣���ƽ�������Σ�1�֣�
��4�����Ӿ��壨1�֣���N3?+H2O
 HN3+OH?��2�֣�
HN3+OH?��2�֣���5��������֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ����2�֣�
��6��1��2��1�֣� ��������1�֣� ��112/NA��a3 g��cm-3��2�֣�����

�����������1������ͬ����Ԫ�����ʵݱ�����жϣ������ڵ������У��縺������Ԫ���Ƿ�����һ��������С��Ԫ����ﮣ���2����̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p3��L������Ų�ͼΪ
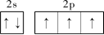 ����3�����ݵȵ�����ĸ����жϣ���N3����Ϊ�ȵ�����ķ���ΪCO2��N2O�����ݼ۲���ӶԻ������ۣ�NO3��������ԭ�������Լ۵��ӣ��ռ乹��Ϊƽ�������Σ���4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ���Ӿ��壻��������Ϊǿ�������Σ����е������ˮ�⣬ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��N3��+H2O
����3�����ݵȵ�����ĸ����жϣ���N3����Ϊ�ȵ�����ķ���ΪCO2��N2O�����ݼ۲���ӶԻ������ۣ�NO3��������ԭ�������Լ۵��ӣ��ռ乹��Ϊƽ�������Σ���4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ���Ӿ��壻��������Ϊǿ�������Σ����е������ˮ�⣬ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��N3��+H2O HN3+OH������5��N2O�е㣨��88��49�棩��NH3�е㣨��33��34�棩�ͣ�����Ҫԭ���ǰ�����֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ������6���ٵ������к��е�������������1���Ҽ��ͺ�2���м�����Ŀ֮��Ϊ1��2����������Ϊ�������壬���ڵĻ�ѧ������Ϊ����������������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������ȱ�پ����ṹ�����н�����
HN3+OH������5��N2O�е㣨��88��49�棩��NH3�е㣨��33��34�棩�ͣ�����Ҫԭ���ǰ�����֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ������6���ٵ������к��е�������������1���Ҽ��ͺ�2���м�����Ŀ֮��Ϊ1��2����������Ϊ�������壬���ڵĻ�ѧ������Ϊ����������������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������ȱ�پ����ṹ�����н�����
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 �����幹���� ��
�����幹���� ��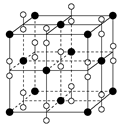
 ��ÿ��COCl2�����ں���________���Ҽ���________���м���������ԭ�Ӳ�ȡ________�ӻ������ʽ��
��ÿ��COCl2�����ں���________���Ҽ���________���м���������ԭ�Ӳ�ȡ________�ӻ������ʽ��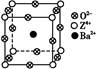

 �Ŀռ乹��Ϊ��������,���以Ϊ�ȵ������һ�ַ���Ϊ����������
�Ŀռ乹��Ϊ��������,���以Ϊ�ȵ������һ�ַ���Ϊ���������� 