��Ŀ����
��(Ga)����(Ge)����(As)����(Se)��Ϊ�������ڵ�Ԫ�أ������ڸ߿Ƽ���˿�ѧ�ر�����Ϣ�������Ź㷺����;���Իش��������⣺
��1����̬��ԭ�ӵļ۵����Ų�ʽΪ_________________________________��
��2���е�:NH3________AsH3(�������������������ԭ����_____________________��
��3��ij����������׳ơ���˪��������ӽṹ��ͼ��ʾ���û�����ķ���ʽΪ_______,Asԭ�Ӳ�ȡ__________�ӻ���

��4��H2SeO4��H2SeO3���������ֺ����ᣬ����ݽṹ�����ʵĹ�ϵ������H2SeO4��H2SeO3����ǿ��ԭ��________________________��
��5���黯�ؿ���(CH3)2Ga��AsH3��700���·�Ӧ�Ƶã���Ӧ�ķ���ʽΪ_________________���黯�صľ����ṹ����ͼ��ʾ���侧���߳�Ϊa pm����ÿ�������þ�����������Ԫ�ص�����Ϊ____________g(��NA��ʾ�����ӵ�������ֵ����

��1����̬��ԭ�ӵļ۵����Ų�ʽΪ_________________________________��
��2���е�:NH3________AsH3(�������������������ԭ����_____________________��
��3��ij����������׳ơ���˪��������ӽṹ��ͼ��ʾ���û�����ķ���ʽΪ_______,Asԭ�Ӳ�ȡ__________�ӻ���

��4��H2SeO4��H2SeO3���������ֺ����ᣬ����ݽṹ�����ʵĹ�ϵ������H2SeO4��H2SeO3����ǿ��ԭ��________________________��
��5���黯�ؿ���(CH3)2Ga��AsH3��700���·�Ӧ�Ƶã���Ӧ�ķ���ʽΪ_________________���黯�صľ����ṹ����ͼ��ʾ���侧���߳�Ϊa pm����ÿ�������þ�����������Ԫ�ص�����Ϊ____________g(��NA��ʾ�����ӵ�������ֵ����

��1��4s24p2��1�֣�
��2������1�֣�д�����ڡ����÷֣� NH3���Ӽ�������������NH3�е����AsH3 (2�֣�ֻҪָ��NH3���Ӽ�����������)
��3��As4O6��1�֣� sp3��1�֣�
��4��H2SeO4��H3SeO3�ɱ�ʾΪ(HO)2SeO2��(HO)3Se��H2SeO3�е�SeΪ+4�ۣ���H2SeO4�е�SeΪ+6�ۣ�����Se-O-H�е�O�ĵ��Ӹ���Seƫ�ƣ�Խ�����H+(2�֣������𰸼���)
��5��(CH3)3Ga + AsH3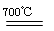 GaAs + 3CH4��2�֣���д��Ӧ�������۷֣���ѧʽд��������ƽ���÷֣���
GaAs + 3CH4��2�֣���д��Ӧ�������۷֣���ѧʽд��������ƽ���÷֣���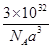 (2�֣������𰸼���)
(2�֣������𰸼���)
��2������1�֣�д�����ڡ����÷֣� NH3���Ӽ�������������NH3�е����AsH3 (2�֣�ֻҪָ��NH3���Ӽ�����������)
��3��As4O6��1�֣� sp3��1�֣�
��4��H2SeO4��H3SeO3�ɱ�ʾΪ(HO)2SeO2��(HO)3Se��H2SeO3�е�SeΪ+4�ۣ���H2SeO4�е�SeΪ+6�ۣ�����Se-O-H�е�O�ĵ��Ӹ���Seƫ�ƣ�Խ�����H+(2�֣������𰸼���)
��5��(CH3)3Ga + AsH3
 GaAs + 3CH4��2�֣���д��Ӧ�������۷֣���ѧʽд��������ƽ���÷֣���
GaAs + 3CH4��2�֣���д��Ӧ�������۷֣���ѧʽд��������ƽ���÷֣���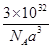 (2�֣������𰸼���)
(2�֣������𰸼���)�����������1�����ǵ������ڵڢ�A��Ԫ�أ�ԭ��������32���������ݺ�������Ų����ɿ�֪����̬��ԭ�ӵļ۵����Ų�ʽΪ4s24p2��
��2�����ڵ�Ԫ�صķǽ�����ǿ�����NH3���Ӽ�������������NH3�е����AsH3�е㣻
��3�����ݷ��ӽṹ��֪�������ں���6����ԭ�ӣ�4����ԭ�ӣ���ѧʽΪAs4O6������ÿ��Asԭ���γ�3�����ۼ�������Ϊ����ԭ�ӻ���1�Թ¶Ե��ӣ����Բ��õ���sp3�ӻ���
��4������H2SeO4��H3SeO3�ɱ�ʾΪ(HO)2SeO2��(HO)3Se��H2SeO3�е�SeΪ+4�ۣ���H2SeO4�е�SeΪ+6�ۣ�����Se-O-H�е�O�ĵ��Ӹ���Seƫ�ƣ�Խ�����H+������H2SeO4��H2SeO3����ǿ��
��5���黯�ؿ���(CH3)2Ga��AsH3��700���·�Ӧ�Ƶã������ԭ���غ��֪������һ���������Ǽ��飬��÷�Ӧ�ķ���ʽΪ(CH3)3Ga + AsH3
 GaAs + 3CH4�����ݾ����ṹ��֪GaAs�����У�ÿ��As��4��Ga��������Asԭ��ȫ���ھ������棬������4���������߳�Ϊa pm�����Ծ��������
GaAs + 3CH4�����ݾ����ṹ��֪GaAs�����У�ÿ��As��4��Ga��������Asԭ��ȫ���ھ������棬������4���������߳�Ϊa pm�����Ծ����������a3pm3������ÿ���������þ����ĸ�����
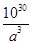 ����˺��е�Asԭ�Ӹ�����
����˺��е�Asԭ�Ӹ�����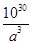 ��4�����ʵ�����
��4�����ʵ����� mol������AsԪ�ص�������
mol������AsԪ�ص������� mol��75g/mol��
mol��75g/mol��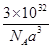 g��
g��
��ϰ��ϵ�д�
�����Ŀ
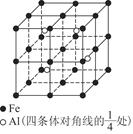
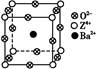

 Ϊ̼ԭ�ӣ�
Ϊ̼ԭ�ӣ� Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
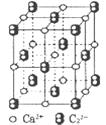
 �Ŀռ乹��Ϊ��������,���以Ϊ�ȵ������һ�ַ���Ϊ����������
�Ŀռ乹��Ϊ��������,���以Ϊ�ȵ������һ�ַ���Ϊ���������� 
 ��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�