��Ŀ����
X��Y��Z��R��W��Ϊ���ڱ���ǰ�����ڵ�Ԫ�أ���ԭ��������������X2����Y������ͬ�ĺ�������Ų���Z����̬�⻯��ķе������һ����ͬ��Ԫ����̬�⻯��ķе�ͣ�R�Ļ�̬ԭ����ǰ������Ԫ�صĻ�̬ԭ���е���������ࣻWΪ����Ԫ�أ�X��W�γɵ�ij�ֻ�������Z���⻯���Ũ��Һ����ʱ��Ӧ������ʵ������ȡZ�����嵥�ʡ��ش��������⣨��ػش����Ԫ�ط��ű�ʾ����
��1��R�Ļ�̬ԭ�ӵĺ�������Ų�ʽ��____________��
��2��Z���⻯��ķе������һ����ͬ��Ԫ���⻯��ķе�͵�ԭ����________________________________________________________________________��
��3��X��Z�е縺�Խϴ����________��Z��ij�ֺ������γ�����ʵ������ȡX�γɵĵ��ʣ���������ӵĿռ乹��Ϊ________����������������ѧ����������________��X��Z��X�ļ���________109��28�䣨���������������������֪���µ��Ӷ�֮��ij������µ��Ӷ���ɼ����Ӷ�֮��ij������ɼ����Ӷ�֮��ij�������
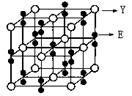
��4��X��Y�γɵĻ�����Y2X�ľ�����ͼ������X���ӵ���λ��Ϊ________����һ��X���Ӿ�����������е�Y����Ϊ����ļ�����Ϊ________���û�������MgO��ȣ��۵�ϸߵ���________��

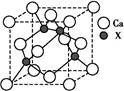
��5����֪�û�����ľ����߳�Ϊa pm����û�������ܶ�Ϊ________g��cm��3��ֻҪ���г���ʽ�����ؼ������ֵ�������ӵ���������ֵΪNA����
��1��R�Ļ�̬ԭ�ӵĺ�������Ų�ʽ��____________��
��2��Z���⻯��ķе������һ����ͬ��Ԫ���⻯��ķе�͵�ԭ����________________________________________________________________________��
��3��X��Z�е縺�Խϴ����________��Z��ij�ֺ������γ�����ʵ������ȡX�γɵĵ��ʣ���������ӵĿռ乹��Ϊ________����������������ѧ����������________��X��Z��X�ļ���________109��28�䣨���������������������֪���µ��Ӷ�֮��ij������µ��Ӷ���ɼ����Ӷ�֮��ij������ɼ����Ӷ�֮��ij�������
��4��X��Y�γɵĻ�����Y2X�ľ�����ͼ������X���ӵ���λ��Ϊ________����һ��X���Ӿ�����������е�Y����Ϊ����ļ�����Ϊ________���û�������MgO��ȣ��۵�ϸߵ���________��

��5����֪�û�����ľ����߳�Ϊa pm����û�������ܶ�Ϊ________g��cm��3��ֻҪ���г���ʽ�����ؼ������ֵ�������ӵ���������ֵΪNA����
��1��1s22s22p63s23p63d54s1��[Ar]3d54s1
��2��HF���Ӽ�����������HCl���Ӽ䲻�������
��3��O�������Ρ����ۼ������Լ�����λ�����ɣ�����
��4��8��������������塡MgO
��5��
��2��HF���Ӽ�����������HCl���Ӽ䲻�������
��3��O�������Ρ����ۼ������Լ�����λ�����ɣ�����
��4��8��������������塡MgO
��5��

��1�����ݺ��ع������֪ǰ�������л�̬ԭ���е����������Ҳ���۲�����Ų�ʽΪ3d54s1����������Ϊ6������RΪ��Ԫ�أ����̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d54s1��[Ar]3d54s1����2����������Ϣ����֪Ԫ��X��Y��Z��W�ֱ�ΪO��Na��Cl��Mn����HF���Ӽ�����������HCl���Ӽ䲻����������ʷе㣺HF��HCl����3��O��Cl�е縺�Խϴ����O��Cl�ĺ�������������ʵ������ȡ��������ΪKClO3��ClO3-�ļ۲���Ӷ���Ϊ4��3���Ҽ���1���µ��Ӷԣ�����ClO3-�Ŀռ乹�ͣ�VSEPRģ��Ϊ�����壩Ϊ�����Σ�ClO3-��Cl��O��Ϊ���ۼ�����������ԭ���ϴ���һ�Թµ��Ӷԣ��µ��ӶԵ��ų������ڳɼ����Ӷԣ���O��Cl��O�ļ���С��109��28�䡣��4���ɾ����ṹ��֪O2���������п�������λ��Ϊ8����һ��O2�����������Na��Ϊ�����γɵļ�����Ϊ�����壻����Mg2�������������Na���࣬�����Ӱ뾶С��Na�������Ӱ뾶�����MgO�ľ����ܴ���Na2O�ģ����۵㣺MgO��Na2O����5�����á���̯������֪һ�������к���4��Na2O�����ʸû�������ܶ�Ϊ g��cm��3��ע��1 pm��10��10 cm����
g��cm��3��ע��1 pm��10��10 cm����
 g��cm��3��ע��1 pm��10��10 cm����
g��cm��3��ע��1 pm��10��10 cm����
��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ

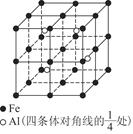
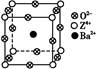
 Ϊ̼ԭ�ӣ�
Ϊ̼ԭ�ӣ� Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��

