��Ŀ����
����Ŀ�����ܴ���һ����ָ������ת��Ϊ���ܻ�ѧ�ܽ��д��棬����̫���⡢CO2��H2O���ɼ״��Ĺ��ܴ���װ����ͼ��ʾ���Ʊ���ʼʱ���ӽ���Ĥ�������Һ������ȣ�������������ȷ����
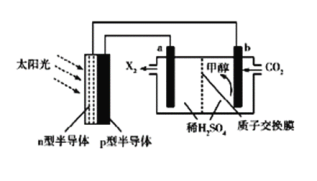
A. b���ĵ缫��ӦʽΪCO2+6e-+6H+=CH3OH+H2O
B. n�Ͱ뵼��Ϊ����
C. X2ΪO2��H+��b����a���ƶ�
D. ���Ʊ�32g�״�ʱ�����ӽ���Ĥ������Һ���104g
���𰸡�C
��������
A.b���ĵ缫��ӦʽΪCO2+6e-+6H+=CH3OH+H2O,Ϊ��ԭ��Ӧ,����A��ȷ��
B.��ͼ��b�������״�����֪��,b��Ϊ����������n�Ͱ뵼��Ϊ��������B��ȷ��
C.a��Ϊ�������缫��ӦΪ2H2O-4e-=O2![]() 4H+��H+��a����b���ƶ�,��C����
4H+��H+��a����b���ƶ�,��C����
D.�ɵ�ʧ�����غ�ɵù�ϵʽ:6H2O![]() 3O2
3O2![]() 2CH3OH��������32gCH3OHʱ��a������54gˮ,b��������CH3OH�⣬������18gˮ�������ӽ���Ĥ������Һ���104g����D��ȷ��
2CH3OH��������32gCH3OHʱ��a������54gˮ,b��������CH3OH�⣬������18gˮ�������ӽ���Ĥ������Һ���104g����D��ȷ��
�����ΪC��

 �п������п��Ծ����ϵ�д�
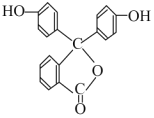
�п������п��Ծ����ϵ�д�����Ŀ���ױ�(![]() )��һ����Ҫ�Ļ���ԭ�ϣ���������������ȩ(
)��һ����Ҫ�Ļ���ԭ�ϣ���������������ȩ(![]() )��������(
)��������(![]() )�Ȳ�Ʒ���±��г����й����ʵIJ����������ʣ���ش�
)�Ȳ�Ʒ���±��г����й����ʵIJ����������ʣ���ش�
���� | ��״ | �۵㣨�棩 | �е㣨�棩 | ����ܶ� ����ˮ=1g/cm3�� | �ܽ��� | |
ˮ | �Ҵ� | |||||
�ױ� | ��ɫҺ����ȼ�ӷ� | -95 | 110.6 | 0.8660 | ���� | ���� |
����ȩ | ��ɫҺ�� | -26 | 179 | 1.0440 | �� | ���� |
������ | ��ɫƬ״����״���� | 122.1 | 249 | 1.2659 | �� | ���� |
ע���ױ�������ȩ�������������ܡ�
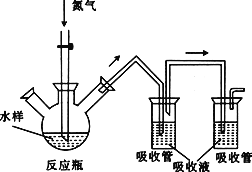
ʵ���ҿ�����ͼװ��ģ���Ʊ�����ȩ��ʵ��ʱ��������ƿ�м���0.5g��̬�����Դ������ټ���15mL������(��Ϊ�ܼ�)��2mL�ױ�������������70�棬ͬʱ��������12mL�������⣬�ڴ��¶��½��跴Ӧ3Сʱ��

��1��װ��a��������__________________����Ҫ������____________________��
��2������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________���˷�Ӧ��ԭ�������������Ͽɴ�___________����ԭ��������=(���������������/ȫ����Ӧ���������)��100%��
��3�����ⶨ����Ӧ�¶�����ʱ���ױ���ת�����������¶ȹ���ʱ������ȩ�IJ���ȴ�������٣����ܵ�ԭ����________________________________________________��
��4����Ӧ��Ϻ�Ӧ���Һ������Ȼ��ȴ������ʱ����Ӧ���� ________��________(���������)�Ȳ��������ܵõ�����ȩ�ֲ�Ʒ��
��5��ʵ���м���������������ҷ�Ӧʱ��ϳ�����ʹ����ȩ��Ʒ�в����϶�ı����ᡣ����ӻ��б�����ı���ȩ�з���������ᣬ��ȷ�IJ���������_______(������˳������ĸ)��
a.�Ի��Һ���з�Һ b.���ˡ�ϴ�ӡ�����
c.���м����������pH��2 d.��������̼��������Һ�����