��Ŀ����
����Ŀ�����ܱ���Ϊ21������߷�չDZ���������Դ���������ʹ���������������õ���Ҫ�о�����
��.������A(H3BNH3)��һ��DZ�ڵĴ�����ϣ�������Ԫ��״����(HB=NH)3ͨ�����·�Ӧ�Ƶã�3CH4+2(HB=NH)3+6H2O=3CO2+6H3BNH3
��ش��������⣺
��1����̬Bԭ�ӵļ۵����Ų�ʽΪ___��B��C��N��O��һ�������ɴ�С��˳��Ϊ___��CH4��H2O��CO2�ļ��ǰ����ɴ�С��˳������Ϊ___��
��2����(HB=NH)3��Ϊ�ȵ�������л�����Ϊ___(�����ʽ)��
��.�����İ�ȫ���������������Ӧ�õĹؼ���
��1��ӡ�����³�Ƚ���ѧ�о����ĵ�Datta��Pati���˽���ADF������һ�����ͻ�ϩ�ഢ�����(C16S8)�����о��������۽Ƕ�֤�����ֲ��ϵķ��ӳ�ƽ��ṹ(��ͼ1)��ÿ���ӻ�ƽ������������������10��H2���ӡ�
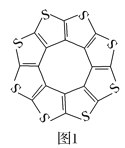
��C16S8������Cԭ�Ӻ�Sԭ�ӵ��ӻ�������ͷֱ�Ϊ___��
����ؼ������������ʾ��
��ѧ�� | C��S | C=S | C16S8��̼��� |
����/pm | 181 | 155 | 176 |
�ӱ������ݿ��Կ�����C16S8��̼�����������C��S����C=S��֮�䣬ԭ�������___��
��C16S8��H2�������������___��
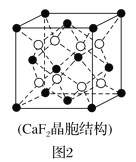
��2�����д���ܵ�ͭ�Ͻ�������������ܶѻ��Ľṹ��������Cuԭ��λ�����ģ�Agԭ��λ�ڶ��㣬��ԭ�ӿɽ��뵽��Cuԭ����Agԭ�ӹ��ɵ��������϶�С�����Cuԭ����Agԭ�ӵ�ͬ�������þ��崢���ľ����ṹ��CaF2(�����ṹ��ͼ2)���ƣ��þ��崢���Ļ�ѧʽΪ___��
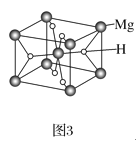
��3��MgH2�ǽ����⻯�ﴢ����ϣ��侧����ͼ3��ʾ����֪�þ�����ܶ�Ϊag��cm-3���������Ϊ___cm3(�ú�a��NA�Ĵ���ʽ��ʾ��NA��ʾ�����ӵ�������ֵ)��
���𰸡�2s22p1 N>O>C>B CO2>CH4>H2O C6H6 sp2��sp3 C16S8�����е�̼�������һ���̶ȵ�˫������ ���»��� Cu3AgH8 ![]()
��������
��. (1) ��̬Bԭ�ӵļ۵���Ϊ��2s��2p�ܼ��ϵĵ��ӣ����ݹ���ԭ����д��ԭ�Ӽ۵����Ų�ʽ��ͬһ����Ԫ�ص�һ����������ԭ��������������������ƣ����ǵ�IIA�塢��V A���һ�����ܴ���������Ԫ�أ�CH4��H2O��CO2�Ŀռ乹�ͷֱ����������塢V�Ρ�ֱ���Σ��ų������µ��Ӷ�֮����ų���>�µ��ӶԺͳɼ����Ӷ�֮����ų���>�ɼ����Ӷ�֮����ų�����
(2)��(HB=NH) 3��Ϊ�ȵ���������к���12��ԭ�ӡ��۵�������30��
II. (1) ��C16S8������Cԭ�Ӻ�Sԭ�ӵļ۲���ӶԸ����ֱ���3��4�����ݼ۲���ӶԻ��������ж�ԭ���ӻ����ͣ�
��C16S8�����е�̼�������һ���̶ȵ�˫�����ʣ�
�۷���֮����ڷ��Ӽ���������
(2) ͭԭ������6��![]() ��3����ԭ������8��
��3����ԭ������8��![]() ��1��Hԭ�Ӹ���Ϊ8��
��1��Hԭ�Ӹ���Ϊ8��
(3) �þ�����Mgԭ������8��![]() ��1��2��Hԭ������2��4��
��1��2��Hԭ������2��4��![]() ��4����þ����к�2��MgH2���������=
��4����þ����к�2��MgH2���������=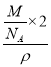 ��
��
��(1).��̬Bԭ�ӵļ۵����Ų�ʽΪ2s22p1��һ������£�ͬ��������Ԫ�ش�����Ԫ�صĵ�һ�����ܳ��������ƣ���������Nԭ�ӵ�2p����ϵĵ��Ӵ��ڰ�������ȶ�״̬���������һ�����ܱ�Oԭ�ӵĴ���ˣ�Ԫ�ص�һ�������ɴ�С��˳��ΪN>O>C>B��CH4�����������η��ӣ�����Ϊ109��28����H2O��V�η��ӣ�����Ϊ105����CO2��ֱ���η��ӣ�����Ϊ180������������ǰ����ɴ�С��˳������ΪCO2>CH4>H2O���ʴ�Ϊ��2s22p1��N>O>C>B��CO2>CH4>H2O��
(2).��(HB=NH)3��Ϊ�ȵ�������л�����ΪC6H6���ʴ�ΪC6H6��
��(1).�ٸ���ͼ1��֪��Cԭ�Ӳ�ȡsp2�ӻ���Sԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��C16S8�����е�̼�������һ���̶ȵ�˫�����ʣ��Ӷ�����C16S8��̼�����������C��S����C===S��֮�䣻�ʴ�Ϊ��C16S8�����е�̼�������һ���̶ȵ�˫�����ʣ�
��C16S8��H2�����������Ϊ���»������ʴ�Ϊ�����»�����
(2).��������֪���þ�����ͭԭ������6��![]() ��3����ԭ������8��
��3����ԭ������8��![]() ��1����ԭ�ӿɽ��뵽��Cuԭ����Agԭ�ӹ��ɵ��������϶�У���Hԭ��λ�ڸþ����ڲ��������þ����к���8��H����þ��崢���Ļ�ѧʽΪCu3AgH8���ʴ�Ϊ��Cu3AgH8��
��1����ԭ�ӿɽ��뵽��Cuԭ����Agԭ�ӹ��ɵ��������϶�У���Hԭ��λ�ڸþ����ڲ��������þ����к���8��H����þ��崢���Ļ�ѧʽΪCu3AgH8���ʴ�Ϊ��Cu3AgH8��
(3).�þ�����Mgԭ������8��![]() ��1��2��Hԭ������2��4��
��1��2��Hԭ������2��4��![]() ��4����þ����к�2��MgH2���þ��������V��
��4����þ����к�2��MgH2���þ��������V��![]() cm3���ʴ�Ϊ��
cm3���ʴ�Ϊ��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���о������仯����Ի�����������Ҫ���塣
(1)��֪����N2(g)+O2(g)=2NO(g) ��H1
��2NH3(g)![]() N2(g)+3H2(g) ��H2
N2(g)+3H2(g) ��H2
��2H2(g)+O2(g)=2H2O(g) ��H3
���Ȼ�ѧ����ʽ��4NH3(g)+6NO(g)=5N2(g)+6H2O(g)��H=__(����H1����H2����H3��ʾ)��
(2)��2L�ܱվ��������У�Ͷ��4mol N2��6mol H2����һ������������NH3����ò�ͬ�¶��£�ƽ��ʱNH3�����ʵ����������±���
�¶�/K | T1 | T2 | T3 | T4 |
n(NH3)/mol | 3.6 | 3.2 | 2.8 | 2.0 |
��������˵���÷�Ӧ�Ѵﵽƽ��״̬����__��
A��3v��(H2)=2v��(NH3) B������������ѹǿ����
C�����������ܶȲ��� D����������¶ȱ��ֲ���
���¶�T1__(����>��<������=��)T3��
����T3�¶��£��ﵽƽ��ʱN2��ת����Ϊ__��
(3)N2O4Ϊ��Ҫ�Ļ���ƽ���֮һ��N2O4��NO2ת�����Ȼ�ѧ����ʽΪN2O4(g)![]() 2NO2(g) ��H��������Ӧ�У�����Ӧ����v��=k����p(N2O4)���淴Ӧ����v��=k����p2(NO2)������k����k��Ϊ���ʳ�������÷�Ӧ�Ļ�ѧƽ�ⳣ��KpΪ__(��k����k����ʾ)������һ����N2O4Ͷ����������к��º�ѹ�ֽ�(�¶�298K��ѹǿ110kPa)����֪��������k��=5��102kPa-1��s-1����N2O4�ֽ�10%ʱ��v��=__kPa��s-1��
2NO2(g) ��H��������Ӧ�У�����Ӧ����v��=k����p(N2O4)���淴Ӧ����v��=k����p2(NO2)������k����k��Ϊ���ʳ�������÷�Ӧ�Ļ�ѧƽ�ⳣ��KpΪ__(��k����k����ʾ)������һ����N2O4Ͷ����������к��º�ѹ�ֽ�(�¶�298K��ѹǿ110kPa)����֪��������k��=5��102kPa-1��s-1����N2O4�ֽ�10%ʱ��v��=__kPa��s-1��
(4)��������������(S2O42-)Ϊ��ԭ���ѳ������е�NO����ͨ�����������װ����ͼ�������ĵ缫��ӦʽΪ__�������еĸ�ĤΪ__(����������������)���ӽ���Ĥ��ÿ����1mol NO����·��ͨ�����ӵ����ʵ���Ϊ__��

����Ŀ������ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ����������֪������ݡ�
��ѧʽ | ����ƽ�ⳣ��(25��) |
HCN | K=4.9��10-10 |
CH3COOH | K=1.8��10-5 |
H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
(1)25 ��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ��������Һ��pH�ɴ�С��˳��Ϊ__________________��
(2)25 ��ʱ��pH=3��CH3COOH��Һ��pH=11��NaOH��Һ��ϣ���������Һ�����ԣ���c(Na��)__________c(CH3COO��)(����>������<������=��)��
(3)NaCN��Һ��ͨ������CO2����������Ӧ�Ļ�ѧ����ʽΪ___________��
(4)25 ��ʱ�� pH=8��CH3COONa��Һ�У�c(Na��)-c(CH3COO-)=___________��
����Ŀ�������й�ˮ��Һ�е�ƽ����ص����⣬�����
��1����֪�����£����Ȼ�����Һ��̼������Һ��ϣ��а�ɫ����������д����Ӧ���ӷ���ʽ______________
��2�������Ϊ100 mL��pH��Ϊ2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ��ͬŨ�ȣ�ͬ�����CH3COONa��NaX��Һ����������Ŀ: CH3COONa��Һ_______NaX��Һ�������������
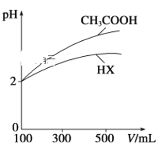
��3������ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ��������������֪��
��ѧʽ | ���볣��(25 ��) |
HCN | K��4.9��10��10 |
CH3COOH | K��1.8��10��5 |
��25��ʱ��Ũ�Ⱦ�Ϊ0.01 mol��L��1 HCN��NaCN�����Һ��_____�ԣ����ᣬ��У�����Һ��HCNŨ��_________CN-Ũ�ȣ������������
��25 ��ʱ����CH3COOH��CH3COONa�Ļ����Һ�У������pH��6������Һ��![]() ��____��
��____��
��4����25��C�£���x mol��L-1�İ�ˮ��y mol��L-1������������ϣ���Ӧ����Һ�������ԣ���c��NH4����____c��Cl����������>�� ����<�� ����=�� �����ú�x��y�Ĵ���ʽ��ʾ����ˮ�ĵ���ƽ�ⳣ��______��