��Ŀ����
15��ij�л���A��C��H��O����Ԫ����ɣ���ȡ21.6g A��������������ȫȼ�գ���ȼ�ղ���ȫ������ͨ����ˮ�Ȼ��ƺͼ�ʯ�Һ�����������������Ϊ14.4g��61.6g�� ������������֪A����Է�������Ϊ108���Իش���1��A�ķ���ʽ��C7H8O��
��2����������ײⶨ��A���ӽṹ�к��б������ǻ�����A���п��ܵĽṹ��ʽ��
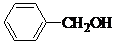 ��
�� ��
�� ��
��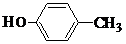 ��
����3����A�����ռӦ���ұ������ж��ֻ������⣬��A���������Ʒ�Ӧ�Ļ�ѧ����ʽ��
 ��
��
���� ��1���Ȼ������ص�����Ϊˮ����������ʯ�����ص�����Ϊ������̼������������n=$\frac{m}{M}$�ֱ������л���A��ˮ�Ͷ�����̼�����ʵ������ٸ��������غ�ȷ��A�ķ���ʽ��
��2�����л�������к��б������ǻ�����Ӧ�û�����1����������Ϊ�����ӣ�Ҳ����Ϊ���״����ݴ�д������ܽṹ��ʽ��
��3���ܹ����ռӦ��˵��Ϊ�����ӣ��������ж��ֻ������⣬���ݣ�2�������ּ�����ȷ����ṹ��ʽ����д����Ӧ�ķ���ʽ��
��� �⣺��1��21.6gA�����ʵ���Ϊ��$\frac{21.6g}{108g/mol}$=0.2mol����ʯ�����ص�Ϊ������̼���������������̼�����ʵ���Ϊ��$\frac{61.6g}{44g/mol}$=1.4mol�����л�������к���Cԭ�ӵ���ĿΪ��$\frac{1.4mol}{0.2mol}$=7��
�Ȼ������ص�Ϊˮ����������ˮ�����ʵ���Ϊ��$\frac{14.4g}{18g/mol}$=0.8mol��˵��0.2mol���л�������к�����ԭ�ӵ����ʵ���Ϊ��0.8mol��2=1.6mol������л�������к���Hԭ����Ϊ��$\frac{1.6mol}{0.2mol}$=8��
���л�������к�����ԭ����ĿΪ��$\frac{108-12��7-1��8}{16}$=1��
���Ը��л���ķ���ʽΪ��C7H8O��
�ʴ�Ϊ��C7H8O��
��2������ʽΪC7H8O���л�������к��б������ǻ�������Ϊ������࣬��Ϊ���࣬��Ϊ�����ӣ����ڵĽṹ�У� ��
�� ��
�� ����Ϊ���ֻ࣬��Ϊ���״���
����Ϊ���ֻ࣬��Ϊ���״���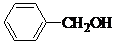 ��
��
�ʴ�Ϊ��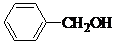 ��
�� ��
�� ��
�� ��
��
��3����A�����ռӦ���ұ������ж��ֻ������⣬��AΪ�����ӣ����б����Ϻ���2��H��Ϊ�� ��A������������Һ��Ӧ�Ļ�ѧ����ʽΪ��
��A������������Һ��Ӧ�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ�ע�����������غ㶨����ȷ���л������ʽ�е�Ӧ�ã���ȷ�����л���ṹ������Ϊ���ؼ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | CH3CH3�е�����̼ԭ����BF3�е���ԭ�Ӿ���ȡsp2�ӻ� | |
| B�� | ������ʯӢ�����еĹ�ԭ�Ӿ���ȡsp3�ӻ� | |
| C�� | BeCl2�е���ԭ�Ӻ�H2O�е���ԭ�Ӿ���ȡsp�ӻ� | |
| D�� | CO2�е�̼ԭ����CH2=CH2�е�����̼ԭ�Ӿ���ȡsp�ӻ� |
| A�� | �ߴ��ȵĶ�������㷺�����������ά�����ά��ǿ��ᡰ��·�� | |
| B�� | ��K2FeO4����Cl2��������ˮ������ɱ���������ã�Ҳ������ˮ���� | |
| C�� | ��ɫ��ѧ�ĺ�����Ӧ�û�ѧԭ���Ի�����Ⱦ�������� | |
| D�� | �ҹ������ձ����ȱ����ƶѪ�����ڽ������������������Ը��Ʋ�ͬʱ�������ά����C |
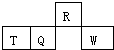 ������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȣ������жϲ���ȷ���ǣ�������
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȣ������жϲ���ȷ���ǣ�������| A�� | �����̬�⻯������ȶ��ԣ�R��Q | |
| B�� | ���������ˮ��������ԣ�Q��W | |
| C�� | ԭ�Ӱ뾶��T��Q��R | |
| D�� | R���⻯��ķе����R��ͬ��Ԫ�ص��⻯��ķе� |
| A�� | 0.1 �� b-2a �� mol/L | B�� | 10 �� 2a-b �� mol/L | ||
| C�� | 10 �� b-a �� mol/L | D�� | 10 �� b-2a �� mol/L |
| A�� | 1 mol �� | B�� | 1 mol ���� | C�� | 1 mol ����� | D�� | 1 mol ��ԭ�� |

 ��
��