��Ŀ����
17�������£�������Һ������Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | KAl��SO4��2��Һ��c��SO42-����c��K+��=c��Al3+����c��H+����c��OH-�� | |
| B�� | �÷�̪��ָʾ������ˮ�е�����������ζ��յ㣺c��NH4+����c��Cl-�� | |
| C�� | NH4Cl��Ba��NO3��2�Ļ����Һ��c��Cl-��+2c��NO3-��=c��NH4+��+c��NH3•H2O��+c��Ba2+�� | |
| D�� | ������ˮ�У�c��H+��=2c��ClO-��+c��HClO��+c��OH-�� |
���� A�������Ӳ�ˮ�⣬�����Ӳ���ˮ�⣬��c��K+����c��Al3+����
B����̪�ı�ɫ��ΧΪ8-10���ζ��յ�ʱ��Һ��ʾ���ԣ���c��OH-����c��H+������ϵ���غ��֪c��NH4+����c��Cl-����
C�����ݻ��Һ�е������غ��жϣ�
D�����ݷ�Ӧ����Һ�е������غ�͵���غ������
��� �⣺A��KAl��SO4��2��Һ�У�������ˮ�����Һ��ʾ���ԣ���c��OH-����c��H+��������Һ������Ũ�ȴ�СΪ��c��SO42-����c��K+����c��Al3+����c��H+����c��OH-������A����
B���÷�̪��ָʾ������ˮ�е�����������ζ��յ㣬���ڷ�̪�ı�ɫ��ΧΪ8-10���ζ��յ�ʱ��Һ��ʾ���ԣ���c��OH-����c��H+������ϵ���غ��֪��c��NH4+����c��Cl-������B����
C��NH4Cl��Ba��NO3��2�Ļ����Һ�����������غ�ɵã�c��Cl-��+c��NO3-��=c��NH4+��+c��NH3•H2O��+2c��Ba2+������C����
D��������ˮ�У�������ˮ������Ӧ��Cl2+H2O?HCl+HClO����������ת���ɵ����ʵ������Ȼ���ʹ����ᣬHClΪǿ����ʣ�HClOΪ������ʣ����ݵ���غ�ɵã�c��H+��=c��ClO-��+c��OH-��+c��Cl-�������������غ�ɵã�c��Cl-��=c��ClO-��+c��HClO�������߽�Ͽɵã�c��H+��=2c��ClO-��+c��HClO��+c��OH-������D��ȷ��
��ѡD��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ���������غ㼰����غ�ĺ���Ϊ���ؼ���ע�������ж�����Ũ�ȴ�С���÷�����DΪ�״��㣬��Ҫ���ݷ�Ӧ�ó������غ�c��Cl-��=c��ClO-��+c��HClO����Ϊ�״��㣮

| ѡ�� | ʵ������ | ���� |
| A | ȡijNa2SO3��Һ������������ϡ���ᣬ�������ݣ��ٵμ�BaCl2��Һ��������ɫ������ | Na2SO3�Ѳ��ֱ����� |
| B | ��2mL0.1mol/LNaOH��Һ�еμ�0.1mol/LMgCl2��Һ3�Σ����ְ�ɫ�������ٵμ�3��0.1mol/L FeCl3��Һ�����ֺ��ɫ������ | �ܽ�ȣ�Mg��OH��2��Fe��OH��3 |
| C | ����ˮ��ͨ������SO2����ˮ��ɫ�� | SO2����Ư���� |
| D | ��ͬ�¶��£����Ũ�ȵ�Na2CO3��Na2SO3ˮ��Һ��pH��pH ��Na2CO3����pH��Na2SO3���� | �ǽ�����ǿ����C��S |
| A�� | A | B�� | B | C�� | C | D�� | D |
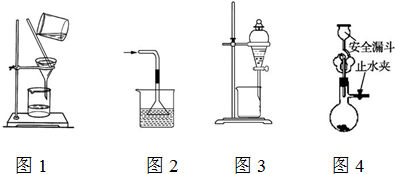
| A�� | ��ͼ1��ʾװ�ý��й��ˣ�����ʱ���Ͻ��� | |
| B�� | ��ͼ2��ʾװ������NH3�ư�ˮ | |
| C�� | ��ͼ3��ʾװ���ñ���ȡ��ˮ�еĵ⣬������ı���Һ��©���¿ڷų� | |
| D�� | ��ͼ4��ʾװ����ʯ��ʯ��ϡ������ȡCO2���� |
| A�� | 0.1 mol•L-1��������Һ��NH4+��Fe2+��Cl-��NO3- | |
| B�� | pH=12����ҺK+��Na+��CH3COO-��Br- | |
| C�� | ������Ӧ����������������Һ��K+��NH4+��HCO3-��Cl- | |
| D�� | ��̪�ʺ�ɫ����Һ��Na+��Fe2+��Cl-��SO42- |
| A�� | Ϊ�˱�֤ʳ��İ�ȫ��ɫ��ζ������Ӧ���з���Ч�Ͷ���ũҩ����ѧʹ��ʳƷ���Ӽ������þ���ϩ���ϴ����а�װ | |
| B�� | ����β����ת��װ�ÿɽ�β���е�NO�� CO���к�����ת��ΪN2��CO2����װ���еĴ����ɽ���NO��CO��Ӧ�Ļ�ܣ���������߸÷�Ӧ��ƽ��ת���� | |
| C�� | ʯ���ѽ����ҪĿ����������͵������͵IJ�����������ʯ�ʹ��ѻ�����ҪĿ���ǵõ��������ϩ����ϩ����̬������ | |
| D�� | �ƹ��Ҵ����ͣ�CO2�IJ����涼���ϵ�̼�������̼���� |
��1��������ˮ��Һ�д��ڵ���ƽ�⣬����0.1mol•L-1����ĵ���ƽ�ⳣ�����±���
| ���� | ����ƽ�ⳣ����25�棩 |
| HClO | K=2.98��10-8 |
| H2CO3 | K1=4.3��10-7 K2=5.6��10-11 |
| H2SO3 | K1=1.54��10-2 K2=1.02��10-7 |
���������ӷ���ʽ���й�˵���������ad
a��������CO2ͨ�����������Һ�У�2ClO-+H2O+CO2=2HClO+CO32-
b��������SO2ͨ��̼������Һ�У�SO2+H2O+2CO32-=2HCO3-+SO32-
c����ͬ�¶�ʱ����pH��������Һ�����ʵ���Ũ�ȹ�ϵ��c��Na2CO3����c��NaClO����c��Na2SO3��
d����ͬ�¶�ʱ�������ʵ�����������������NaOH��Һ��ȫ�к�����NaOH�����Ϊ��V��H2CO3����V��H2SO3����V��HClO��
�������ᣨH2SeO3��Ҳ��һ�ֶ�Ԫ���ᣬ�н�ǿ�������ԣ�����������Һ�в���ͨ��SO2��������ɫ���ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��H2SeO3+2SO2+H2O=Se��+2H2SO4���÷�Ӧ������������H2SO4��
��2����ҵ��ˮ�г�����һ������Cr2O72-��CrO42-�����Ƕ����༰��̬ϵͳ�����ܴ���������д������ŷţ�
���ڷ�ˮ�д���ƽ�⣺2CrO42-����ɫ��+2H+?Cr2O72-����ɫ��+H2O���ı�����ʹ����ƽ��������Ӧ�����ƶ���������˵����ȷ����ac
a��ƽ�ⳣ��Kֵ���Բ��ı�
b���ﵽ��ƽ��CrO42-���������ʵ���Cr2O72-����������
c���ٴ�ƽ��ǰ����Ӧ����һ�������淴Ӧ����
d��ƽ���ƶ���ﵽ��ƽ����ҺpHһ������
��Cr2O72-��CrO42-�������ɵ�Cr��OH��3����Һ�д������³����ܽ�ƽ�⣺Cr��OH��3��s��?Cr3+��aq��+3OH?��aq�������£�Cr��OH��3���ܶȻ�Ksp=c��Cr3+��•c3��OH-��=10-32����c��Cr3+������10-3 mol•L-1����Һ��pH����4ʱ��û�У���С���û�С�������������
��3����֪����CO��g��+2H2��g��?CH3OH��g��
��2CH3OH��g��?CH3OCH3��g��+H2O��g��
��CO��g��+H2O��g��?CO2��g��+H2��g��
ij�¶���������Ӧ��ƽ�ⳣ����ֵ����Ϊa1��a2��a3������¶��·�Ӧ 3CO��g��+3H2��g��?CH3OCH3��g��+CO2��g�� �Ļ�ѧƽ�ⳣ��K=a12•a2•a3L4•mol-4���ú�a1��a2��a3�Ĵ���ʽ��ʾ����
��ij�̶�������ܱ������м���3molCO��3molH2����ַ�Ӧ��ָ���ԭ���¶ȣ��ⶨ������ѹǿΪ��Ӧǰ��$\frac{2}{3}$����CO��ת����50%��
| A�� | 0.1 mol/L NaHCO3��Һ��0.1 mol/L NaOH��Һ�������ϣ�������Һ�У�c��Na+����c��C${{O}_{3}}^{2-}$����c��HC${{O}_{3}}^{-}$����c��OH-�� | |
| B�� | 20 mL 0.1 mol/L CH3COONa��Һ��10 mL 0.1 mol/L�����Ϻ�����ԣ�������Һ�У�c��CH3COO-����c��Cl-����c��CH3COOH����c��H+�� | |
| C�� | �����£�pH=2��������pH=12�İ�ˮ�������ϣ�������Һ�У�c��Cl-����c��H+����c��N${{H}_{4}}^{+}$����c��OH-�� | |
| D�� | 0.1 mol/L CH3COOH��Һ��0.1 mol/L NaOH��Һ�������ϣ�������Һ�У�c��OH-����c��H+��+c��CH3COOH�� |
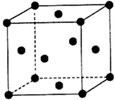 W��X��Y��Z��W��X��Y��Z�ֱ����Ԫ�ط��ţ���ΪԪ�����ڱ���ǰ�����ڵ�Ԫ�أ���ԭ������������������W��X��YΪ������Ԫ�أ����ǵĵ�����ͨ��״���¾�Ϊ��ɫ���壮XԪ�صĻ�̬ԭ����ͬ����Ԫ�ػ�̬ԭ���к��е�δ�ɶԵ�������࣬YԪ�صĻ�̬ԭ����s�ܼ��ϵĵ���������p�ܼ��ϵĵ�������ZΪ����Ԫ�أ����̬ԭ���Ǿ���4s1�ṹ�Ļ�̬ԭ��������������ԭ�ӣ��Իش����и��⣺
W��X��Y��Z��W��X��Y��Z�ֱ����Ԫ�ط��ţ���ΪԪ�����ڱ���ǰ�����ڵ�Ԫ�أ���ԭ������������������W��X��YΪ������Ԫ�أ����ǵĵ�����ͨ��״���¾�Ϊ��ɫ���壮XԪ�صĻ�̬ԭ����ͬ����Ԫ�ػ�̬ԭ���к��е�δ�ɶԵ�������࣬YԪ�صĻ�̬ԭ����s�ܼ��ϵĵ���������p�ܼ��ϵĵ�������ZΪ����Ԫ�أ����̬ԭ���Ǿ���4s1�ṹ�Ļ�̬ԭ��������������ԭ�ӣ��Իش����и��⣺