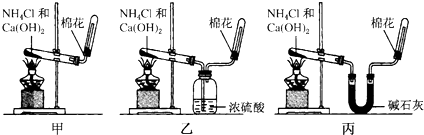
��Ŀ����
3��ij�����Һ�п��ܺ���HCl��MgCl2��AlCl3��NH4Cl��Na2CO3��KCl�е�һ�ֻ������ʣ�������Һ����μ���NaOH��Һ���������������ʵ�����n��������NaOH��Һ�����V���Ĺ�ϵ��ͼ��ʾ���ش��������⣺��1����Һ��һ�����е�������HCl��AlCl3��NH4Cl��һ�������е�������Na2CO3��MgCl2���ѧʽ����
��2����Һ�п��ܺ��е��������Ȼ��أ������ƣ����жϸ������Ƿ���ڵ�ʵ�鷽������ɫ��Ӧ������������ɫ�ܲ����۲���ɫ��ӦΪ��ɫ��
��3���ֱ�д��AB�Ρ�BC�η�����Ӧ�����ӷ���ʽ��
��AB��ΪAl3++3OH-�TAl��OH��3������BC��ΪNH4++OH-=NH3•H2O��
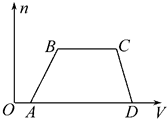
���� ����ͼ���֪��OA��������˵����HCl������Na2CO3��
AB���г�����CD�γ�����ȫ��ʧ��˵������ΪAl��OH��3��˵����Һ��ֻ��AlCl3����MgCl2��
BC�γ������䣬˵����Һ�к���NH4Cl���˶�笠����������������ӷ�Ӧ��
���ݷ�����֪����Һ��һ������HCl��AlCl3��NH4Cl��һ������Na2CO3��MgCl2�����ܺ���KCl���ݴ˽��н��
��� �⣺����ͼ���֪��OA��������˵����HCl������Na2CO3��
AB���г�����CD�γ�����ȫ��ʧ��˵������ΪAl��OH��3��˵����Һ��ֻ��AlCl3����MgCl2��
BC�γ������䣬˵����Һ�к���NH4Cl���˶�笠����������������ӷ�Ӧ��
���ݷ�����֪����Һ��һ������HCl��AlCl3��NH4Cl��һ������Na2CO3��MgCl2�����ܺ���KCl��
��1����Һ��һ������HCl��AlCl3��NH4Cl����Һ��һ������Na2CO3��MgCl2���ʴ�Ϊ��HCl��AlCl3��NH4Cl��Na2CO3��MgCl2��
��2����Һ�п��ܴ��ڵ�����Ϊ�Ȼ��أ���֤�����ӵĴ�����Ҫ������ɫ��Ӧ�����մ���Һ��������ɫ�ܲ����۲���ɫ��ӦΪ��ɫ��˵�������Ȼ��أ��ʴ�Ϊ���Ȼ��أ���ɫ��Ӧ������ɫ�ܲ����۲���ɫ��ӦΪ��ɫ��
��3����AB�������������������ӷ�Ӧ��������������������Ӧ�����ӷ���ʽΪ��Al3++3OH-=Al��OH��3�����ʴ�Ϊ��Al3++3OH-=Al��OH��3����
��BC��笠����������������ӽ������һˮ�ϰ�����Ӧ�����ӷ���ʽΪ��NH4++OH-=NH3•H2O���ʴ�Ϊ��NH4++OH-=NH3•H2O��
���� ���⿼����δ֪����ƶϣ���Ŀ�Ѷ��еȣ���ȷͼ�������߱仯�ĺ���Ϊ���ؼ���������ؿ���ѧ���ķ���������������ע�����ճ������ʵ����ʼ����鷽������ȷ�����������������ӷ�Ӧ������Ϊ�������ͻ�ƿڣ�

| A�� | C2H4 | B�� | C2H2 | C�� | C6H6 | D�� | C3H8 |
| A�� | CH4��C2H4 | |
| B�� | CH3-CH=CH-CH3 �� CH3-CH2-CH2-CH3 | |
| C�� | O2��O3 | |
| D�� | C2H6��C3H8 |
| A�� | �������ظ������Һ�м��������Ҵ���3COH3CH2OH+2Cr2O72-+13H+��4Cr3++3CH3COO-+11H2O | |
| B�� | AlCl3•6H2O��SOCl2��ϲ����ȣ�AlCl3•6H2O+3SOCl2$\frac{\underline{\;\;��\;\;}}{\;}$Al��OH��3+3SO2��+9HCl | |
| C�� | SbCl3ˮ�⣺SbCl3+H2O?SbOCl+2HCl | |
| D�� | �ڵ�����Ȼ�̼��Һ�м�Ũ��KI��Һ��I2+3I-?I3- |