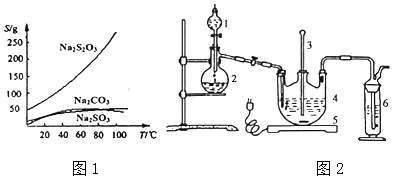
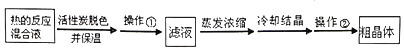
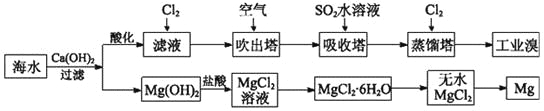
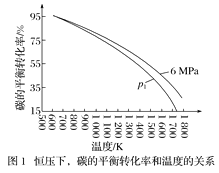
��Ŀ����
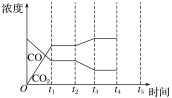
����Ŀ����֪N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4kJ/mol��һ����������ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ������˵����ȷ���ǣ� ��
2NH3(g) ��H=-92.4kJ/mol��һ����������ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ������˵����ȷ���ǣ� ��
A.ǰ20�����ڷ�Ӧ�ų�������Ϊ46.2kJ
B.ʱ����������ʼͶ�ŵ�����Ũ������ԭ����2������Ӧ���ת��������ƽ�ⳣ������
C.��25���Ӹı�����������ǽ�NH3�ӷ�Ӧ��ϵ�з����ȥ
D.����60����ʱ��Ӧ�ִﵽ��ƽ�⣬��ʱ��ı������һ��������ѹǿ
���𰸡�BC
��������
A��Ҫ��֪�����ȶ��پͱ���֪���μӷ�Ӧ��H2�����ʵ�����������c=n��V��ͼ����ʾ����c��V������������ķ��ȶ��٣���A����
B����������������ʼͶ�ŵ�����Ũ������ԭ����2�����൱������ѹǿ��ƽ�������ƶ�����Ӧ���ת���������¶Ȳ��䣬ƽ�ⳣ�����ֲ��䣬��B��ȷ��
C����25����ʱ��������������Ũ�Ȳ��䣬������Ũ����1.00mol/L��Ϊ0��˵���ı�������ǽ�NH3�ӷ�Ӧ��ϵ�з����ȥ����C��ȷ��
D�������͵�����Ũ�ȼ�С��������Ũ������˵��ƽ�������ƶ�������Ӧ�Ƿ��ȷ�Ӧ��������ʹƽ�������ƶ�����ʱ��ı�������ǽ����¶ȣ���D����
�ʴ�ѡBC��

 Ӧ������ҵ��ϵ�д�
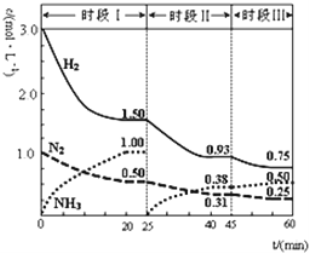
Ӧ������ҵ��ϵ�д�����Ŀ����ѧ�������������ء�������������Ѫ�쵰�ס����쵰����O2��ϻ��Ƶ�����о����ٶ��价���¶Ⱦ�Ϊ36.8�档
(1)Ѫ�쵰��Hb���O2�γɶ���Ѫ�����ڷ�Ӧ�٣�HbH+(aq)+O2(g)![]() HbO2(aq)+H+(aq)���÷�Ӧ���Է����У�������H______0(���������)��ѪҺ�л�ͬʱ���ڷ�Ӧ�ڣ�CO2+H2O
HbO2(aq)+H+(aq)���÷�Ӧ���Է����У�������H______0(���������)��ѪҺ�л�ͬʱ���ڷ�Ӧ�ڣ�CO2+H2O![]() H++HCO3-����Ϸ�Ӧ�٢ڣ��β�����ѹ_____(��ϸߡ��ϵ͡�)������CO2�ų����⣬�ӻ�ѧƽ��ǶȽ���ԭ�� ____________��
H++HCO3-����Ϸ�Ӧ�٢ڣ��β�����ѹ_____(��ϸߡ��ϵ͡�)������CO2�ų����⣬�ӻ�ѧƽ��ǶȽ���ԭ�� ____________��
(2)�����д������쵰�� MbҲ�ɽ��O2�γ�MbO2������Ӧ�ۣ�Mb(aq)+O2(g)![]() MbO2(aq)����ƽ�ⳣ��K=
MbO2(aq)����ƽ�ⳣ��K=![]() �������������䣬��������ѹp(O2)����Kֵ___(��������С�����䡱)����֪������ѹp(O2)=2.00 kPa ��ƽ����ϵ�У�
�������������䣬��������ѹp(O2)����Kֵ___(��������С�����䡱)����֪������ѹp(O2)=2.00 kPa ��ƽ����ϵ�У�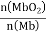 =4.0������Ŀ�����p(O2)=21 kPa�������ʱ Mb������������϶�(ƽ��ת����)ԼΪ_______________(������λ��Ч����)��
=4.0������Ŀ�����p(O2)=21 kPa�������ʱ Mb������������϶�(ƽ��ת����)ԼΪ_______________(������λ��Ч����)��
(3)Hb���Ӿ����ĸ��ǻ�����ÿ���ǻ������ֹ���(T�ͺ�R��)��ͼ�У�T0��R0��ʾδ���O2��T�ͺ�R�ͣ��Ҵ��ڿ���ı乹ЧӦ��T0![]() R0������ƽ�ⳣ��ΪK0�����ķ���O2��Hb���ĸ��ǻ���Ϻ�T4
R0������ƽ�ⳣ��ΪK0�����ķ���O2��Hb���ĸ��ǻ���Ϻ�T4![]() R4Ҳ�DZ乹ЧӦ������ƽ�ⳣ��ΪK4��
R4Ҳ�DZ乹ЧӦ������ƽ�ⳣ��ΪK4��
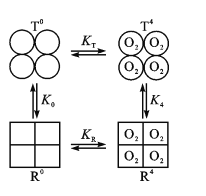
����֪ij���ײ��˷�����T0+4O2![]() T4��Ӧ��n(O2)�������£�
T4��Ӧ��n(O2)�������£�
t/min | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
n(O2)/10-6 mol | 1.68 | 1.64 | 1.58 | 1.50 | 1.40 |
����2.0 min~8.0 min����T�����ʵ����仯��ʾ�ķ�Ӧ����v(T4)Ϊ_________mol��min-1��
���ּٶ�R��Hb��O2�Ľ�ϳ���ΪKR��T��Hb��O2�Ľ�ϳ���ΪKT����֪KR��KT����ͼ��K0____K4(���������)��
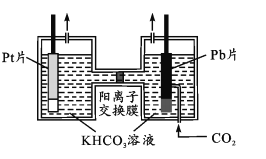
(4)������������ز����ٵ����ʡ�����ͼ��ʾ����PtΪ������Pb(CO2)�����壬ʹCO2�Ϊ��������⾭CO2���ʹ�����KHCO3��Һ��ʹ����������ͬʱ�õ��״�����������ӦʽΪ____���ӵ�����Һ�з���״��IJ���������_______________��
����Ŀ��N2O5��һ��������������һ���¶��·���2N2O5(g)![]() 4NO2(g)��O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ
4NO2(g)��O2(g)����H>0��T1�¶��µIJ���ʵ������Ϊ
t/s | 0 | 500 | 1 000 | 1 500 |
c(N2O5)mol/L | 5.00 | 3.52 | 2.50 | 2.50 |
����˵������ȷ������ ��
A. 500 s��N2O5�ֽ�����Ϊ2.96��10��3 mol/(L��s)
B. T1�¶��µ�ƽ�ⳣ��ΪK1��125��1 000 sʱת����Ϊ50%
C. ������������ʱ��T2�¶��·�Ӧ��1 000 sʱ���N2O5(g)Ũ��Ϊ2.98 mol/L����T1<T2
D. T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1>T2����K1>K2