��Ŀ����
(1)CH4(g )+2O2(g )=CO2(g )+2H2O(g ) ��H=-802.3kJ/mol
���Ȼ�ѧ��Ӧ����ʽ��������_____________________________________��
(2)��֪2g�Ҵ���ȫȼ������Һ̬ˮ�ų�Q kJ��������д����ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ��
��ʽ��____________________________________________________________.
(3)��֪��1mol H-H����1mol N-H����1mol ���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
(4)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C(ʯī��s)+O2(g)=CO2(g) ��H=-393.5kJ/mol ��
2H2(g)+O2(g)=2H2O(l) ��H=-571.6kJ/mol ��
2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l) ��H=-2599kJ/mol ��
���ݸ�˹���ɣ�����298Kʱ��C��ʯī��s����H2(g)����1mol C2H2(g)��Ӧ���ʱ䣺
____________________________.
���Ȼ�ѧ��Ӧ����ʽ��������_____________________________________��
(2)��֪2g�Ҵ���ȫȼ������Һ̬ˮ�ų�Q kJ��������д����ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ��
��ʽ��____________________________________________________________.
(3)��֪��1mol H-H����1mol N-H����1mol
 ���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.(4)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C(ʯī��s)+O2(g)=CO2(g) ��H=-393.5kJ/mol ��
2H2(g)+O2(g)=2H2O(l) ��H=-571.6kJ/mol ��
2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l) ��H=-2599kJ/mol ��
���ݸ�˹���ɣ�����298Kʱ��C��ʯī��s����H2(g)����1mol C2H2(g)��Ӧ���ʱ䣺
____________________________.
��1��1mol���������2mol��������ȫ��Ӧ����1mol������̼��2molˮ����ʱ�ų�802.3KJ����������2��C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ; ��H=-23QKJ�Mmol (3)( 1�M2) N2(g)+( 3�M2) H2(g)=NH3(g) ; ��H=-46KJ�Mmol (4)+226.7KJ�Mmol��
�����������1�����Ȼ�ѧ��Ӧ����ʽ��������1mol���������2mol��������ȫ��Ӧ����1mol������̼��2molˮ����ʱ�ų�����802.3KJ����2���Ҵ�����Է���������46.1mol�Ҵ�������Ϊ46g����Ϊ2g�Ҵ�ȼ������Һ̬ˮ����Q kJ������46g�Ҵ�ȼ������Һ̬ˮʱ�ų�������Ϊ23 Q kJ��������ȼ���ȵ��Ȼ�ѧ����ʽΪ��C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ; ��H=-23QKJ�Mmol����Ӧ���Ƕ��ѻ�ѧ�����յ���������ѧ�����ͷŵ����������N2��H2��Ӧ����1mol NH3(g)�ķ�Ӧ��Ϊ( 1�M2)��946+( 3�M2) ��436-3��391=-46.�ʸ÷�Ӧ���Ȼ�ѧ����ʽ��( 1�M2) N2(g)+( 3�M2) H2(g)=NH3(g) ; ��H="-46KJ�Mmol" (4) �١�2+��1�M2 ������-��1�M2 �����۵æ�H=2��(-393.5)+��1�M2 ����(-571.6)+��1�M2 ����2599=+226.7KJ�Mmol.���÷�Ӧ���Ȼ�ѧ����ʽΪ��2C(ʯī��s)+H2(g)=C2H2(g ) ; ��H=+226.7KJ�Mmol.

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д� ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ

 CH3OCH3(g) + CO2(g)����H��-247kJ/mol
CH3OCH3(g) + CO2(g)����H��-247kJ/mol


 C(s)=
C(s)= CH3OH(g)��H2O(g) ��H����49.0kJ/mol
CH3OH(g)��H2O(g) ��H����49.0kJ/mol
 CH3OH��g������H=��90.8kJ��mol
CH3OH��g������H=��90.8kJ��mol
 H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
 CH3OH(g)+H2O(g)��H=��49.0kJ��mol��1�������¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������¡�����˵����ȷ����
CH3OH(g)+H2O(g)��H=��49.0kJ��mol��1�������¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������¡�����˵����ȷ����


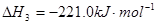
 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0

 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 
 2CO2+ N2 ��H
2CO2+ N2 ��H

