��Ŀ����
3��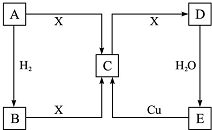 ��֪A�ǵ��ʣ�A��B��C��D��E 5�����ʾ���ͬһ��Ԫ�أ�X�ǵؿ��к�������Ԫ���γɵĵ��ʣ����ǵ��ת����ϵ��ͼ��ʾ���Իش��������⣮
��֪A�ǵ��ʣ�A��B��C��D��E 5�����ʾ���ͬһ��Ԫ�أ�X�ǵؿ��к�������Ԫ���γɵĵ��ʣ����ǵ��ת����ϵ��ͼ��ʾ���Իش��������⣮��1��ͨ������£���AΪ���壬C��D���Ǵ�����Ⱦ�
��д�����з�Ӧ�Ļ�ѧ����ʽ��D��E3NO2+H2O=NO+2HNO3��
�ڹ�ҵ����ȡB�Ļ�ѧ����ʽ��N2+3H2$?_{���¸�ѹ}^{����}$2NH3��
��ʵ�����м���B�IJ���������ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ������
�ܱ�״���£���ʢ��D���Թܵ�����ʢ��ˮ��ˮ���У�һ��ʱ��ٶ����ʲ���ɢ�����Թ���������Һ�����ʵ���Ũ��Ϊ0.045mol/L��
��2��ͨ������£���AΪ����ɫ���壺
��д��B����Һ��C��Ӧ�Ļ�ѧ����ʽ��2H2S+SO2=3S��+2H2O��
�ڽ�Cͨ����ˮ�е���������ˮ��ɫ��������Ӧ�����ӷ���ʽ��SO2+Br2+2H2O=4H++SO42-+2Br-��
���� X�ǵؿ��к�������Ԫ���γɵĵ��ʣ�ӦΪO2������ת����ϵ��ӦΪS��N2��
��1��ͨ������£���AΪ���壬C��D���Ǵ�����Ⱦ���AΪN2��BΪNH3��CΪNO��DΪNO2��EΪHNO3��
��2��ͨ������£���AΪ����ɫ���壬ӦΪS����BΪH2S��CΪSO2��DΪSO3��EΪH2SO4����϶�Ӧ���ʵ������Լ���ĿҪ������⣮
��� �⣺X�ǵؿ��к�������Ԫ���γɵĵ��ʣ�ӦΪO2������ת����ϵ��ӦΪS��N2��
��1��ͨ������£���AΪ���壬C��D���Ǵ�����Ⱦ���AΪN2��BΪNH3��CΪNO��DΪNO2��EΪHNO3��
��D��EΪ����������ˮ������ķ�Ӧ������ʽΪ3NO2+H2O=NO+2HNO3��
�ʴ�Ϊ��3NO2+H2O=NO+2HNO3��
�ڹ�ҵ���õ����������ϳɰ�����ȡB�Ļ�ѧ����ʽΪN2+3H2$?_{���¸�ѹ}^{����}$2NH3��
�ʴ�Ϊ��N2+3H2$?_{���¸�ѹ}^{����}$2NH3��
�۰���Ϊ�������壬��ˮ��Ӧ����NH3•H2O�������ӳ�OH-���ӣ���Һ�ʼ��ԣ�����ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����˵���а������ɣ�
�ʴ𰸣���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ������
�ܼ�����������ݻ�Ϊ3L����������������Ϊ3L������������ˮ��Ӧ�ķ���ʽΪ��
3NO2+H2O=2HNO3+NO��
3��22.4L 1��22.4L
3L 1L
��Ӧǰ������������3L��Ϊ1L��������Һ�����Ϊ2L������Һ������Ϊ���ᣬ
3NO2+H2O=2HNO3+NO��
3��22.4L 2mol
3L $\frac{5}{56}$mol
������������ʵ���Ũ��ΪC=$\frac{n}{V}$=0.045mol/L��
�ʴ�Ϊ��0.045mol/L��
��2��ͨ������£���AΪ����ɫ���壬ӦΪS����BΪH2S��CΪSO2��DΪSO3��EΪH2SO4��
��B��CΪH2S��SO2�ķ�Ӧ������ʽΪ2H2S+SO2=3S��+2H2O��
�ʴ�Ϊ��2H2S+SO2=3S��+2H2O��
�ڶ���������л�ԭ�ԣ�������ˮ����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪSO2+Br2+2H2O=4H++SO42-+2Br-���ɹ۲쵽��ˮ��ɫ��
�ʴ�Ϊ����ˮ��ɫ��SO2+Br2+2H2O=4H++SO42-+2Br-��
���� ���⿼���������ƶϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬�������ʵ���ɫ�����ʵ����ʽ����ƶϣ�A��������������˵��A�д��ڵ�ij��Ԫ���ж��ֻ��ϼۣ��ٽ��E�����ʷ��������Ŀ�ѶȲ���

| A�� | ��ˮ��Ӧ�����Ҵ� | B�� | ����ˮ��Ӧʹ֮��ɫ | ||
| C�� | ��������Ӧ�������� | D�� | ��������Ӧ���ɶ�����̼��ˮ |
| A�� | H2O2�������� | B�� | H2O2ʧȥ���� | C�� | ��Ԫ�ر���ԭ | D�� | I-���������� |
| A�� | ���������һ���м��� | B�� | ��������п����Ǽ�������� | ||
| C�� | ���������һ��û�б��� | D�� | ��������п�������ϩ |
| A�� | ����������������ԭ��Ӧ����������ԭ�� | |
| B�� | �ǽ���������������ԭ��Ӧ�������������� | |
| C�� | ��ԭ����������ԭ��Ӧ��ʧȥ1�����ӣ�����ԭ��ʧȥ3�����ӣ������ƵĻ�ԭ��С���� | |
| D�� | ����Ԫ�ر���ԭʱһ�����ɽ������� |
| A�� | v��NH3��=0.003 mol•��L•s��-1 | B�� | v��NO��=0.08 mol•��L•s��-1 | ||
| C�� | v��H2O��=0.003 mol•��L•s��-1 | D�� | v��O2��=0.01 mol•��L•s��-1 |
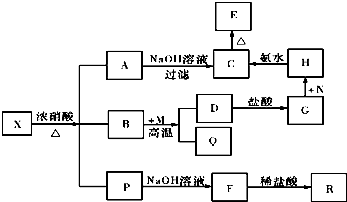
 X�Ļ�ѧʽΪFe3C��P������Ļ�ѧʽΪCO2��NO2��
X�Ļ�ѧʽΪFe3C��P������Ļ�ѧʽΪCO2��NO2��