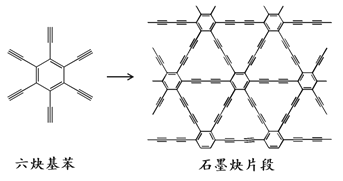
��Ŀ����
����Ŀ���廯��ͭ��һ�ְ�ɫ��ĩ����������ˮ������ˮ�л���ⶼ��ֽ⣬�ڿ����л�������������ɫ��ĩ���Ʊ�CuBr��ʵ�鲽�����£�
����1.����ͼ��ʾ��������ƿ�м���45gCuSO4��5H2O��19gNaBr��150mL��й�������ˮ��60��ʱ���Ͻ��裬���ʵ�����ͨ��SO2 2Сʱ��
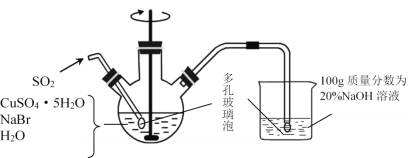
����2.��Һ��ȴ����ȥ�ϲ���Һ���ڱܹ�������¹��ˡ�
����3.��������������SO2��ˮ����������SO2���Ҵ���������ϴ�ӡ�
����4.��˫����������ֱ�װ��Ũ������������ƣ��и���3��4h���پ������������������ո��
��1��ʵ����������ˮ�辭��У����Ŀ���dz�ȥ����ˮ�е�______________��д��ѧʽ����
��2������1�У���������ƿ�з�Ӧ����CuBr�����ӷ���ʽΪ_______________��
�ڿ��Ʒ�Ӧ��60����У�ʵ���пɲ�ȡ�Ĵ�ʩ��_____________��
��˵����Ӧ����ɵ�������__________________��
��3������2������Ҫ�ܹ��ԭ����_______________��
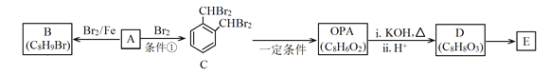
��4������3��ϴ�Ӽ���������SO2����ԭ����_____________________����ܼ��������ѵ�Ŀ����________________________________��
��5������������װ���ձ��е�����Һ���������Ҫ��Na2SO3��NaHSO3�ȣ���ȡ�ϴ�����Na2SO3��7H2O���塣�벹��ʵ�鲽�裨���õ�SO2�������ڸ�ƿ�У���20%NaOH��Һ���Ҵ���
��_______________________________________________��
��__________________________________________________��
�ۼ�������ά����C��Һ����������������Ũ������ȴ�ᾧ��
��__________________________________________________��
�ݷ���ո������и��
���𰸡�O2 2Cu2+ + 2Br��+ SO2 + 2H2O �� 2CuBr��+SO42��+4H+ 60��ˮԡ���� ��Һ��ɫ��ȫ��ȥ ��ֹCuBr����ֽ� ��ֹCuBr������ ��ȥ�����Ҵ�����ʹ������ٸ��� ���ձ��м���ͨ��SO2������ Ȼ�����ձ��м���100g 20%��NaOH��Һ ���ˣ����Ҵ�ϴ��2��3��
��������
(1)�廯��ͭ�ܱ���������������Ҫ�ų������ĸ��ţ�
(2)��������ƿ��ͭ���ӱ���������ԭ����ͭ���ӣ��������ӷ�Ӧ����CuBr������
�ڿ��Ʒ�Ӧ��60�����У�������60����ˮԡ���ȣ�
��45gCuSO45H2OΪ0.18mol��19gNaBrΪ0.184mol������NaBr�Թ��������Ե���Һ�е�ͭ����������ʱ��Ӧ����ɣ�
(3)�廯��ͭ�����ֽ⣻
(4)�廯��ͭ�ڿ����л�������������ϴ�Ӽ���������SO2�����Է�ֹCuBr���������ܼ��������ѿ��Գ�ȥ�����Ҵ�����ʹ������ٸ��
(5)�ձ��е�����Һ��Ҫ��Na2SO3��NaHSO3�ȣ���ȡ�ϴ�����Na2SO37H2O���壬�������ձ��м���ͨ��SO2�����ͣ���Na2SO3����NaHSO3��������Ԫ���غ��֪����ʱ��Һ��NaHSO3�����ʵ���Ϊ0.5mol��Ȼ�����ձ��м���100g 20%��NaOH��Һ��ʹNaHSO3ǡ����ȫ��Ӧ����Na2SO3����������ά����C��Һ(������)������Ũ������ȴ�ᾧ�����ˣ����Ҵ�ϴ��2��3�Σ���ȥ������������ʣ�����ո������и���ݴ˴��⡣
(1)�廯��ͭ�ܱ����������������ö�������ԭͭ���������廯��ͭҪ�ų������ĸ��ţ�����ͨ����еķ�����ȥ����ˮ�е�O2���ʴ�Ϊ��O2��
(2)��������ƿ��ͭ���ӱ���������ԭ����ͭ���ӣ��������ӷ�Ӧ����CuBr��������Ӧ�����ӷ���ʽΪ2Cu2++2Br+SO2+2H2O=2CuBr��+SO42+4H+���ʴ�Ϊ��2Cu2++2Br+SO2+2H2O=2CuBr��+SO42+4H+��
�ڿ��Ʒ�Ӧ��60�����У�������60����ˮԡ���ȣ��ʴ�Ϊ�� 60��ˮԡ���ȣ�
��45gCuSO45H2OΪ0.18mol��19gNaBrΪ0.184mol������NaBr�Թ��������Ե���Һ�е�ͭ����������ʱ��Ӧ����ɣ�����˵����Ӧ����ɵ���������Һ��ɫ��ȫ��ȥ���ʴ�Ϊ����Һ��ɫ��ȫ��ȥ��
(3)�廯��ͭ�����ֽ⣬���Բ���2������Ҫ�ܹ⣬��ֹCuBr����ֽ⣬�ʴ�Ϊ����ֹCuBr����ֽ⣻
(4)�ڿ����л�����������������ϴ�Ӽ���������SO2�����Է�ֹCuBr������������ܼ��������ѿ��Գ�ȥ�����Ҵ�����ʹ������ٸ���ʴ�Ϊ����ֹCuBr����������ȥ�����Ҵ�����ʹ������ٸ��
(5)�ձ��е�����Һ��Ҫ��Na2SO3��NaHSO3�ȣ���ȡ�ϴ�����Na2SO37H2O���壬�������ձ��м���ͨ��SO2�����ͣ���Na2SO3����NaHSO3��������Ԫ���غ��֪����ʱ��Һ��NaHSO3�����ʵ���Ϊ0.5mol��Ȼ�����ձ��м���100g20%��NaOH��Һ��ʹNaHSO3ǡ����ȫ��Ӧ����Na2SO3����������ά����C��Һ(������)������Ũ������ȴ�ᾧ�����ˣ����Ҵ�ϴ��23�Σ���ȥ������������ʣ�����ո������и���ʴ�Ϊ�����ձ��м���ͨ��SO2�����ͣ�Ȼ�����ձ��м���100g20%��NaOH��Һ�����ˣ����Ҵ�ϴ��23�Ρ�

����Ŀ����������п��һ�ֶ���Ե����������ϣ�ijС���Դ�����п��������ͭ�������Ϊԭ��ģ�ҵ������������п���������£�
��֪����������������pH��Χ���±���ʾ��
Zn��OH��2 | Fe��OH��2 | Fe��OH��3 | Cu��OH��2 | |
��ʼ����pH | 5.4 | 7.0 | 2.3 | 4.7 |
��ȫ����pH | 8.0 | 9.0 | 4.1 | 6.7 |
����������
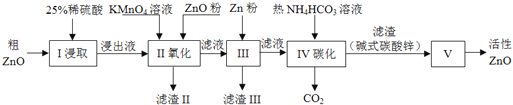
��1������I������25%ϡ�������98%Ũ���ᣨ�ܶ�Ϊ1.84g/mL�����ƣ���������������������ձ��⣬����Ҫ_______��ѡ���ţ�
A��������ƽ B����Ͳ C������ƿ D����ͷ�ι�
��2������II��ͨ������KMnO4����������ZnO����pH�����Գ�ȥ�������ʣ���������Ƿ���ȫ��ʵ�������_________������pH�����˷�Χ��_________��
��3������III�м���Zn�۵������ǣ���________���ڽ�һ��������ҺpH��
��4������IV��ʹ����NH4HCO3��Һ�ܴٽ�Zn2+ת��Ϊ���������¶Ȳ��˹��ߣ���ԭ�������________��
��5������V��_______�����������ƣ��н��У���֪��ʽ̼��п�Ļ�ѧʽΪZn5��OH��6��CO3��2����д��������Ӧ�Ļ�ѧ����ʽ��________���ж��ѷֽ���ȫ�IJ�����________��
��6�������·����ⶨ���û�������п�Ĵ��ȣ�
��ȡ1.000g��������п����15.00mL 1.000mol/L������Һ��ȫ�ܽ�
����Ũ��Ϊ0.500mol/L�ı�����������Һ�ζ�ʣ�����ᣬ�����յ�ʱ��������������Һ12.00mL��
�������ʲ����뷴Ӧ�������û�������п�Ĵ���Ϊ_______�����ڵζ�ʱ��ʵ�ʲ����й�����Σ�1mL��ҺΪ25�Σ����εζ���������Ϊ_____��
����Ŀ����ˮ����ͭ�ڼ�����650��ʱ��ʼ�ֽ���������ͭ�����壮ij�С��ͨ��ʵ�飬̽����ͬ�¶�������������ɣ�ʵ��װ�����£�
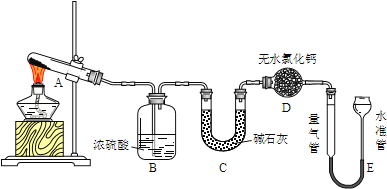
ÿ��ʵ�����ⶨB��C�����ĸı��E���ռ�������������ʵ���������£�E�������������������״������
ʵ����� | �¶� | ��ȡCuSO4����/g | B��������/g | C��������/g | E���ռ�������/mL |
�� | T1 | 0.640 | 0.320 | 0 | 0 |
�� | T2 | 0.640 | 0 | 0.256 | V2 |
�� | T3 | 0.640 | 0.160 | Y3 | 22.4 |
�� | T4 | 0.640 | X4 | 0.192 | 33.6 |
��1��ʵ�������A�е�������______��D����ˮ�Ȼ��Ƶ�������_______��
��2���ڲ���E���������ʱ��Ӧע����_______��Ȼ�����ˮ���������ܵ�Һ����ƽ����ˮ����Һ����������ܣ�����������______������ƫ��������ƫС����������������
��3��ʵ�����B�����յ�������_____��ʵ�����E���ռ�����������______��
��4���Ʋ�ʵ�����CuSO4�ֽⷴӦ����ʽΪ��_______��
��5�����ݱ������ݷ�����ʵ�����������C���ӵ�����Y3=_______g��
��6�����ƽ���ƶ�ԭ�����Ƚ�T3��T4�¶ȵĸߵͲ�˵������________��