��Ŀ����
11��ijƷ���̷۱�����Ϊ�����������β������߳�����ֵ7.8��������ʳ�ÿ����°���NaNO2����ʳ��һ������ζ���ܷ������·�Ӧ��2NaNO2+4HI=2NO+I2+2NaI+2H2O����1��������Ӧ������1.75mol�Ļ�ԭ������������ԭ����������1.75mol��
��2��ij��������Һ�У���2%��5%��NaNO2��ֱ���ŷŻ������Ⱦ�������Լ�����ʹNaNO2ת��Ϊ�����������Ⱦ��N2�������ţ���NaCl����NH4Cl����H2O2����ŨH2SO4
��Ӧ�Ļ�ѧ����ʽΪNaNO2+NH4Cl�TNaCl+N2��+2H2O�����˷�ˮ��ǿ���ԣ��������Һ��pH�ӽ����ԣ��������Ȼ����Һ����������������Ⱦ����Ӧ�ķ���ʽΪNH4++OH-=NH3��+H2O��
��3��������ʵ���Ũ�ȵ�NaOH��Na2CO3�Ļ����Һ�м���ϡ���ᣮ�������ӷ���ʽ����ʵ���������C
A��OH-+CO32-+2H+=HCO3-+H2O B��2OH-+CO32-+3H+=HCO3-+2H2O
C��2OH-+CO32-+4H+=CO2��+3H2O D��OH-+CO32-+3H+=CO2��+2H2O��
���� ��1��2NaNO2+4HI�T2NO+I2+2NaI+2H2O�У�NԪ�صĻ��ϼ۽��ͣ�IԪ�صĻ��ϼ����ߣ�
��2��NaNO2ֱ���ŷŻ������Ⱦ����Ҫ���л�ԭ�Ե����ʽ����������ɵ�����
��3�������ʵ���Ũ�ȵ�NaOH��Na2CO3�Ļ����Һ�к�������������̼���Ƶ����ʵ�����ȣ�ϡ������뵽NaOH��Na2CO3�Ļ����Һ�У����������������Ʒ�������кͣ�ʣ�����������̼���Ʒ�����Ӧ����������ʱ������ӦCO32-+H+=HCO3-���������ʱ������Ӧ��CO32-+2H+=CO2��+H2O���Դ������
��� �⣺��1��2NaNO2+4HI�T2NO+I2+2NaI+2H2O�У�NԪ�صĻ��ϼ۽��ͣ�IԪ�صĻ��ϼ����ߣ���NaNO2Ϊ���������������뻹ԭ�������ʵ���֮��Ϊ1��1����1.75mol�Ļ�ԭ������������ԭ����������1.75mol��
�ʴ�Ϊ��1.75��
��2��NaNO2ֱ���ŷŻ������Ⱦ����Ҫ���л�ԭ�Ե����ʽ����������ɵ�������ѡ���Ȼ�識��ɣ�����NaNO2+NH4Cl�TNaCl+N2��+2H2O������ѡ����ϣ��˷�ˮ��ǿ���ԣ��������Һ��pH�ӽ����ԣ��������Ȼ����Һ������NH4++OH-=NH3��+H2O����������������Ⱦ��
�ʴ�Ϊ���ڣ�NaNO2+NH4Cl�TNaCl+N2��+2H2O��NH4++OH-=NH3��+H2O��
��3��A����NaOH��Na2CO3�����ʵ�����Ϊ1mol������������������ȷ�����Ӧ��OH-+H+=H2O��1mol������������1mol���ᣬ�ٷ�����ӦCO32-+H+=HCO3-������������ʽ��ӵã�OH-+CO32-+2H+=HCO3-+H2O����A��ȷ��
B����NaOH��Na2CO3�����ʵ�����Ϊ2mol������������������ȷ�����Ӧ��2OH-+2H+=2H2O��2mol������������2mol���ᣬ�ٷ�����ӦCO32-+H+=HCO3-�������֮����1mol̼���ƣ�����������ʽ��ӵã�2OH-+CO32-+3H+=HCO3-+2H2O����B��ȷ��
C����NaOH��Na2CO3�����ʵ�����Ϊ2mol��2mol������������2mol�����ӣ�ʣ��2mol��������2mol̼���Ʒ�Ӧ����2mol̼��������ӣ����ӷ���ʽӦΪ2OH-+2CO32-+4H+=2HCO3-+2H2O����OH-+CO32-+2H+=HCO3-+H2O����C����
D����NaOH��Na2CO3�����ʵ�����Ϊ1mol�����������������ȷ�����Ӧ��OH-+H+��H2O��1mol������������1mol���ᣬ�ٷ�����ӦCO32-+2H+=CO2��+H2O������������ʽ��ӵã�OH-+CO32-+3H+=CO2��+2H2O����D��ȷ��
�ʴ�Ϊ��C��
���� ���⿼��������ԭ��Ӧ�����ӷ�Ӧ��Ϊ��Ƶ���㣬��ȷ��Ӧ��Ԫ�صĻ��ϼ۱仯�ǽ����Ĺؼ������ػ�������Ŀ��飬ע��֪ʶǨ��Ӧ�ã���Ŀ�ѶȲ���

 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�| ʵ�鲽�� | �й����� |
| �ټ�������Na2SO4������ | ��Ҫ����Na2SO4������Ϊ14.2�� |
| �ڳ���Na2SO4���� | ������Ҫ�õ�����Ҫ�����ǣ�������ƽ��ҩ�� |
| �۽�Na2SO4����100mL�ձ��У�����������ˮ | Ϊ�˼ӿ��ܽ����ʣ����Բ�ȡ��Щ��ʩ�� �ò��������� |
| �ܽ��ձ�����Һת����500mL����ƿ�� | Ϊ�˷�ֹ��Һ������Ӧ��ȡʲô��ʩ�� ת�ƹ������ò����������� |
| ��������ƿ�м�����ˮ���̶��� | �ڽ��д˲���ʱ����ˮ���̶���1-2���״�Ӧ��β����� ���ý�ͷ�ιܼ�ˮ����Һ����̶������� |
��2��ȡ����Na2SO4��Һ10mL��ˮϡ�͵�100mL��ϡ�ͺ���Һ��Na+�����ʵ���Ũ����0.04mol/L��
��3�������Тݲ�����ʱ������ˮ�����̶��ߣ������������ƣ�
��4������Na2SO4��Һʱ�����в��������ʹ���ƫ�ߵ���C��
A����Һǰ������ƿ������������ˮ
B���ܽ����ʱ��Һ��ɽ�
C������ʱ��������ƿƿ���̶���
D�����ݺ�������ƿҡ�Ⱦ�ƽ�ž��ã�Һ����ڿ̶��ߣ��ټ�ˮ���ݣ�
����֪��Na2S2O3+H2SO4=Na2SO4+S��+SO2+H2O��ij�о�С�����ݸ÷�Ӧ̽����������Է�Ӧ���ʵ�Ӱ�죬���ʵ�����£�
| ʵ�� ��� | ʵ���¶� /�� | Na2S2O3 | H2SO4 | ����ˮ��� /mL | ||
| ���/mL | Ũ��/mol•L-1 | ���/mL | Ũ��/mol•L-1 | |||
| �� | 25 | 10 | 0.1 | 10 | 0.1 | 0 |
| �� | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
| �� | 25 | 5 | 0.2 | 10 | 0.2 | 5 |
| �� | 50 | 5 | 0.1 | 10 | 0.1 | 5 |
| �� | 50 | 10 | 0.2 | 5 | 0.2 | 5 |
A��ʵ��ٺ͢�̽��������������ʱNa2S2O3Ũ�ȶ���ط�Ӧ���ʵ�Ӱ��
B��ʵ��ٺ͢���Һ����ǵ�ʱ����ͬ
C��������������ʱ��̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬Ӧѡ��ʵ��ۺ͢�
D����ͬѧ��ʵ���в��õ��о�������ʵ��ȽϷ�
��ʵ������SO2ͨ��Na2S��Na2CO3�Ļ����Һ�����Ʊ���������ƣ���Ӧԭ��Ϊ��
2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2
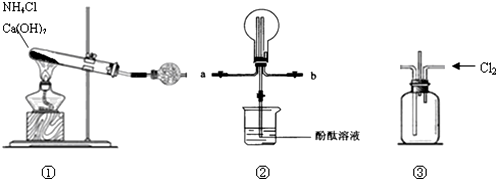
��ʵ���Na2S����Ҫ��ϸߣ�����ͼ1��ʾ��װ�ÿɽ���ҵ����Na2S�ᴿ����֪Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ����ᴿ����Ϊ�����ѳ����õĹ�ҵ��Na2S����Բ����ƿ�У�����һ�������ľƾ�������ˮ����ͼ1��ʾװ��������������������ͨ����ȴˮ��ͬʱˮԡ���ȣ�����ƿ�й��岻�ټ���ʱ��ֹͣ���ȣ�����ƿȡ�£��������ȹ��ˣ�����ȴ�ᾧ�����ˣ������ù���ϴ�ӡ�����õ�Na2S•9H2O���壮
��1�����ᴿ�����С����ȹ��ˡ�������Ŀ���Ƿ�ֹ���ƽᾧ��������ʧ��ȥ�����ʣ�
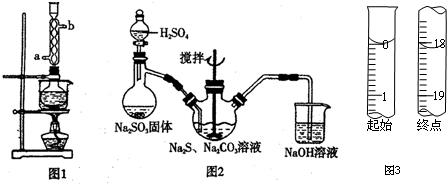
��2����ͼ2��ʾװ����ȡNa2S2O3������ʢ��Na2SO3����IJ�������������������ƿ��NaOH��Һ������������SO2��β������ֹ��Ⱦ��
��3�����շ���Ʒ��Na2S2O3•5H2O�Ĵ��ȣ�������������ͨ��������ԭ�ζ����ⶨ����ط�Ӧ����ʽΪ2Na2S2O3+I2=2NaI+Na2S4O6��ȷ��ȡW��g��Ʒ����ƿ�У�����������ˮ�ܽ⣬���μӵ�����Һ��ָʾ������0.1000��mol•L?1��ı���Һ���еζ���
��ش�
�ٵ���ζ��յ�ı�־�������һ�ε�ı���Һ����Һ��Ϊ��ɫ����30s�ڲ��ָ�ԭɫ��
�ڵζ���ʼ���յ��Һ��λ����ͼ3�������ĵ�ı���Һ���Ϊ18.10mL����Ʒ�Ĵ���Ϊ$\frac{0.362M}{W}$%����Na2S2O3•5H2O��Է�������ΪM����
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ��Na2S2O3•5H2O�Ĵ��ȵIJ������ƫ�ͣ��ƫ�ߡ�����ƫ�͡����䡱����
| A�� | ������ʹ����KMnO4��Һ��ɫ��������������ƣ���˱�Ϊ������ | |
| B�� | ���Ľṹ��ʽΪ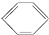 ������˫����������ˮ�����ӳɷ�Ӧ ������˫����������ˮ�����ӳɷ�Ӧ | |
| C�� | ����6��̼ԭ�Ӻ�6����ԭ����ͬһƽ���� | |
| D�� | ��1 mL����1 mLˮ��ֻ�Ϻ��ã������� |
| NaCl | MgCl2 | AlCl3 | SiCl4 | ����B | |
| �۵�/�� | 810 | 710 | 190 | -68 | 2 300 |
| �е�/�� | 1 465 | 1418 | 182.7 | 57 | 2 500 |
| A�� | SiCl4�Ƿ��Ӿ��� | B�� | ����B������ԭ�Ӿ��� | ||
| C�� | AlCl3���������� | D�� | KCl���۵����810�� |
| A�� | m+11 | B�� | m+4 | C�� | m-6 | D�� | m-5 |
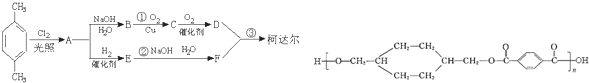
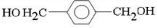 ��D
��D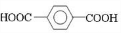 ��E
��E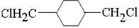
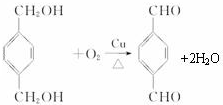 ����Ӧ����������Ӧ
����Ӧ����������Ӧ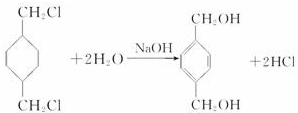 ����Ӧ����ȡ����Ӧ
����Ӧ����ȡ����Ӧ $\stackrel{һ��������}{��}$
$\stackrel{һ��������}{��}$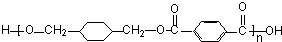 +��2n-1��H2O����Ӧ�������۷�Ӧ��
+��2n-1��H2O����Ӧ�������۷�Ӧ��

 ��
��