��Ŀ����
1��ij��ѧʵ��С��ͬѧ��������װ���Ʊ����ﰱ������̽�����������ʣ�������������ȥ����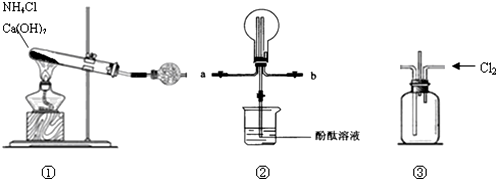
��ش�
��1�������Ʊ������Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+CaCl2+2H2O��
��2���ռ�ʱѡ�����Ľ�����a���a����b����
��3���ڳ����½�����������ͬʱͨ��װ�âۻ�ϼ�������Ӧ������ij�������岢�������̣���д����Ӧ�Ļ�ѧ����ʽ8NH3+3Cl2�TN2+6NH4Cl��
����װ�â��м䵼�ܴ��ݳ���β���к�������Cl2��Ϊ��ֹ��Ⱦ�����ɽ�β��ͨ��ʢ������������Һ��ϴ��ƿ��
���� ��1��ʵ�����ü����Ȼ�����������ƻ�����Ʊ�������
��2�������ܶ�С�ڿ����ܶȣ�Ӧ����������������
��3�����ݷ�Ӧ�����ж�������Ӷ�д����Ӧ�Ļ�ѧ����ʽ�������ܺ�ˮ��Ӧ�����ᣬ��ͼ��ܷ����кͷ�Ӧ�����Կ�������������Һ����������
��� �⣺��1���Ȼ�����������Ʒ�Ӧ�����Ȼ��ơ�������ˮ������ʽΪ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+CaCl2+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+CaCl2+2H2O��
��2�������ܶ�С�ڿ����ܶȣ�Ӧ����������������������a�ڽ��룬��b�ڳ���
�ʴ�Ϊ��a��
��3��������������Ӧ���ɵ��ʵ����Ͱ��̣��������Ȼ�粒��壬��Ӧ����ʽΪ��8NH3+3Cl2�TN2+6NH4Cl�������ܺ�ˮ��Ӧ�����ᣬ��ͼ��ܷ����кͷ�Ӧ�����Կ�������������Һ����������
�ʴ�Ϊ��8NH3+3Cl2�TN2+6NH4Cl������������Һ��
���� ���⿼���˳��������Ʊ�ԭ����װ��ѡ����Ϥ�Ʊ�ԭ���ǽ���ؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
12��100mL 0.3mol•L-1��Na2SO4��Һ��50ml 0.2mol•L-1��Al2��SO4��3��Һ��Ϻ���Һ��SO42-�����ʵ���Ũ��Ϊ��������[��ʾ��ϡ��Һ��Ϻ�������������]��
| A�� | 0.2 mol•L-1 | B�� | 0.4 mol•L-1 | C�� | 0.25 mol•L-1 | D�� | 0.5 mol•L-1 |
16���������ʵķ��������ȷ���ǣ�������
| A | B | C | D | |
| ������ | HCl | NaOH | Cu2��OH��2SO4 | FeSO4•7H2O |
| ������ | H2O | Na2O | CO | Fe3O4 |
| ����� | Ũ���� | ���� | ������Һ | �� |
| A�� | A | B�� | B | C�� | C | D�� | D |
13����Fe��NO3��2��Һ�д�������ƽ�⣺Fe2++2H2O?2Fe��OH��2+2H+������֪Fe2+����ɫΪdz��ɫ���������Һ�м���ϡ���ᣬ����Һ����ɫΪ��������
| A�� | �ޱ仯 | B�� | ��Ϊ��ɫ | C�� | ��Ϊ����ɫ | D�� | ��Ϊ�ػ�ɫ |
11���������ڹ���Ԫ�س���H2O��NH3���γ�����
��1��д��CuԪ�ػ�̬ԭ�ӵĺ�������Ų�1s22s22p63s23p63d104s1��
��2����֪ͭ���ӿ��γ���λ��Ϊ4��������ʢ������ͭˮ��Һ���Թ�����������ˮ���õ�����ɫ��Һ��д���÷�Ӧ�����ӷ���ʽCu2++4NH3•H2O=[Cu��NH3��4]2++4H2O��Cu2++4NH3=[Cu��NH3��4]2+������Һ�м����Ҵ��������������������ɫ���壬ԭ��Ϊ�����İ���ͭ���Ҵ��е��ܽ��ԶС����ˮ�е��ܽ��
��3����C��H��O��S��������Ԫ�ع��ɼס��ҡ������ַ��ӣ�����ԭ�ӵ���Ŀ����Ϊ3��4��8��������18�����ӣ����ҵ���Ҫ�������ʱȽ����£�
��1mol�ҷ��Ӻ���3NA���Ҽ���
�ڱ����ӵ�����ԭ�Ӳ�ȡsp3�ӻ������
�ۼ��ҵ���Է�������������ͬ����������������ʲ������Ҫԭ����H2O2���Ӽ�������������۷е���ߣ�H2O2��ˮ���ӿ��γ������������ˮ����Ȼ��ܣ���Ͼ������ʽ��ͣ���
��1��д��CuԪ�ػ�̬ԭ�ӵĺ�������Ų�1s22s22p63s23p63d104s1��
��2����֪ͭ���ӿ��γ���λ��Ϊ4��������ʢ������ͭˮ��Һ���Թ�����������ˮ���õ�����ɫ��Һ��д���÷�Ӧ�����ӷ���ʽCu2++4NH3•H2O=[Cu��NH3��4]2++4H2O��Cu2++4NH3=[Cu��NH3��4]2+������Һ�м����Ҵ��������������������ɫ���壬ԭ��Ϊ�����İ���ͭ���Ҵ��е��ܽ��ԶС����ˮ�е��ܽ��
��3����C��H��O��S��������Ԫ�ع��ɼס��ҡ������ַ��ӣ�����ԭ�ӵ���Ŀ����Ϊ3��4��8��������18�����ӣ����ҵ���Ҫ�������ʱȽ����£�
| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| �� | 187 | 202 | 2.6 |
| �� | 272.26 | 425.25 | ������Ȼ��� |
�ڱ����ӵ�����ԭ�Ӳ�ȡsp3�ӻ������
�ۼ��ҵ���Է�������������ͬ����������������ʲ������Ҫԭ����H2O2���Ӽ�������������۷е���ߣ�H2O2��ˮ���ӿ��γ������������ˮ����Ȼ��ܣ���Ͼ������ʽ��ͣ���
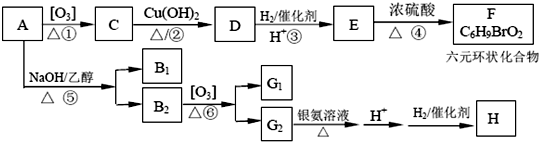
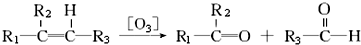
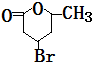
 ��B1 �ķ���ʽΪC6H8��
��B1 �ķ���ʽΪC6H8��
 +H2O��
+H2O��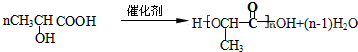 ��
��
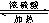 HCOOC2H5+H2O��
HCOOC2H5+H2O��