��Ŀ����
7����ҵ���õ����Σ�����Ϊǿ�ᣩ�Ʊ��ߵ��ᣨH5IO6���������ᣩ�����øߵ��������Խ���������Mn2+����Mn${{O}_{4}}^{-}$����������ͼ��ʾ��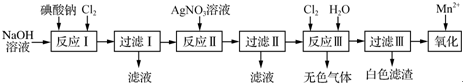
��1����Ӧ���ǽ�����������Ϊ������ˮ��Na2H3IO6���÷�Ӧ�Ļ�ѧ����ʽΪNaIO3+Cl2+3NaOH=Na2H3IO6+2NaCl��
��Ӧ��õ����Dz�����ˮ�ĺ�ɫAg5IO6�����ˢ����Һ���ᣨ��ᡱ��������С����ԣ�
��2����ɫ���������Ϊ��������ɫ�����Ļ�ѧʽΪAgCl��
��3����ҵ��Ϊ���ͳɱ������ٶԻ�������Ⱦ������������Ҫ���Ƽ��������������������ʵ���֮��Ϊn��Cl2����n��AgNO3��=7��10��
��4������Mn2+�����У�����1mol H5IO6ʱ��ת��2mol���ӣ���÷�Ӧ�����ӷ���ʽΪ2Mn2++5H5IO6=2MnO4-+5IO3-+11H++7H2O��
���� ��Ӧ����������������Һ�н�����������Ϊ������ˮ��Na2H3IO6�����˵õ�Na2H3IO6��������������Һ���ɲ�����ˮ�ĺ�ɫAg5IO6�����˵õ���ɫAg5IO6������������ˮ���ɵĴ�����ֽ������������������˵õ��ߵ����øߵ��������Խ���������Mn2+����MnO4-��
��1������������Һ������������������Ϊ������ˮ��Na2H3IO6��ͬʱ�����Ȼ��ƣ�������������Ӧ���ɲ�����ˮ�ĺ�ɫAg5IO6����Һ���������
��2������������֪Ag5IO6���������ɸߵ��ᣬ����������ˮ���ɵĴ�����ֽ��������������������ӽ�������������Ȼ���������
��3�����ݻ�ѧ��Ӧ�Ķ�����ϵ����NaIO3+Cl2+3NaOH=Na2H3IO6+2NaCl��Na2H3IO6+5AgNO3=3HNO3+2NaNO3+Ag5IO6����2Ag5IO6+5Cl2+H2O=10AgCl��+5O2+2H5IO6������õ���
��4������Mn2+�����У�����1mol H5IO6ʱת��2mol���ӣ�H5IO6��IO3-��2e-��Mn2+��MnO4-��5e-����ϵ����غ���ƽ��д���ӷ���ʽ��
��� �⣺��Ӧ����������������Һ�н�����������Ϊ������ˮ��Na2H3IO6�����˵õ�Na2H3IO6��������������Һ���ɲ�����ˮ�ĺ�ɫAg5IO6�����˵õ���ɫAg5IO6������������ˮ���ɵĴ�����ֽ������������������˵õ��ߵ����øߵ��������Խ���������Mn2+����MnO4-��
��1������������Һ������������������Ϊ������ˮ��Na2H3IO6����Ӧ�Ļ�ѧ����ʽΪ��NaIO3+Cl2+3NaOH=Na2H3IO6+2NaCl��ͬʱ�����Ȼ��ƣ�������������Ӧ���ɲ�����ˮ�ĺ�ɫAg5IO6����ӦΪ��Na2H3IO6+5AgNO3=3HNO3+2NaNO3+Ag5IO6������Һ���������ᣬ��Һ�����ԣ�
�ʴ�Ϊ��NaIO3+Cl2+3NaOH=Na2H3IO6+2NaCl���
��2������������֪Ag5IO6���������ɸߵ��ᣬ����������ˮ���ɵĴ�����ֽ�����������������Ӧ�Ļ�ѧ����ʽΪ��2Ag5IO6+5Cl2+H2O=10AgCl��+5O2+2H5IO6�������ӽ�������������Ȼ���������
�ʴ�Ϊ��������AgCl��
��3�����ݻ�ѧ��Ӧ�Ķ�����ϵ���㣺NaIO3+Cl2+3NaOH=Na2H3IO6+2NaCl��Na2H3IO6+5AgNO3=3HNO3+2NaNO3+Ag5IO6����2Ag5IO6+5Cl2+H2O=10AgCl��+5O2+2H5IO6������õ���2Cl2��2Na2H3IO6��10AgNO3��2Ag5IO6��5Cl2����������������Ҫ���Ƽ��������������������ʵ���֮��Ϊn��Cl2����n��AgNO3��=7��10��
�ʴ�Ϊ��7��10��
��4������Mn2+�����У�����1mol H5IO6ʱת��2mol���ӣ�H5IO6��IO3-��2e-��Mn2+��MnO4-��5e-����ϵ����غ���ƽ��д���ӷ���ʽΪ��2Mn2++5H5IO6=2MnO4-+5IO3-+11H++7H2O��
�ʴ�Ϊ��2Mn2++5H5IO6=2MnO4-+5IO3-+11H++7H2O��
���� ���⿼�������ʷ����ᴿ�ķ�������Ҫ�ǻ�ѧ��Ӧ�Ķ�����ϵ�������㣬���̵IJ����жϺͻ�ѧ����ʽ����д�ǽ���ؼ�����Ŀ�ѶȽϴ�

| A�� | ����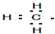 | B�� | CaC2�� | C�� | NH4+��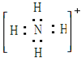 | D�� | �ȷ£�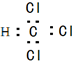 |
| A�� | SF6 | B�� | BeCl2 | C�� | CO2 | D�� | PCl5 |
| A�� | A��B��C��D | B�� | A��C��B��D | C�� | A��C��D��B | D�� | B��D��C��A |
| A�� | ���Ӿ���ľ���������̬�����γ�1mol����ʱ�ų������� | |
| B�� | �������������ܶѻ��������������ܶѻ��Ŀռ���������� | |
| C�� | �ƾ����ṹ��ͼ ���ƾ�����ÿ����ԭ�ӵ���λ��Ϊ8 ���ƾ�����ÿ����ԭ�ӵ���λ��Ϊ8 | |
| D�� | �¶����ߣ������ĵ����Խ����� |



