��Ŀ����
��¯ˮ���Ȼή��ȼ�ϵ������ʡ�Ӱ���¯��ʹ����������������ɰ�ȫ������
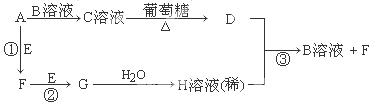
ij��¯ˮ������Ҫ�ɷ���CaCO3��CaSO4��Mg(OH)2��Fe2O3������ϴ���ɳ�ȥ��ˮ���������ԭ������ͼ��ʾ��
��1����ϴʱ��Ϊʹˮ���������ܽ⣬����ѡ�õ��������������� �����ţ���
����
| A������ | B������ | C������ | D��ϡ���� |
��3��ϴ��Һ�е�Fe3+�ḯʴ���ʹܵ�����Ӧ�����ӷ���ʽ���������������������������� ����ˣ�����ϴ��Һ�м������ǿ��ԭ�Ե�SnCl2��Һ����Ӧ�е�Sn2+��Fe3+�����ʵ���֮��Ϊ1��2��Sn2+ת��Ϊ���������������� �������ӷ��ţ���
��4�������ᣨ��H3R��ʾ����������ϴ������Һ��H3R��H2R����HR2����R3���ĺ�����pH�Ĺ�ϵ��ͼ
��ʾ��ͼ��a���������������İٷֺ�������ҺpH�ĸı���仯��ԭ������������������������ ��
������������Һ��pH=4�������ڳ�ȥˮ���е���������pH=4ʱ����Һ������4���������������������������������� ��

��1��D��2��  ��3��
��3�� 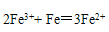
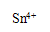
��4�� ����ҺPH����ƽ��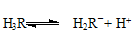 �����ƶ����ԣ�H3R�İٷֺ�����С H2R-
�����ƶ����ԣ�H3R�İٷֺ�����С H2R-
���������������1����ʹ��ϡ�����������������ƣ�����ڷ�Ӧ��ı��棬ʹ��Ӧ�ﲻ����ȫ��Ӧ�ʲ���ѡ���ᡣ��2����ˮ�������ữ֮��ֻ��������Dz��������̼���ƣ��������ܽ�ȸ�С��̼��ƣ�3��Fe3+���������Կ�������л�ԭ�Ե�����Ӧ�����������ӣ��ḯʴ���ʹܵ����ݵ�ʧ����������ȵ�ԭ����Ħ�������������ӣ�����������ӣ��ܹ��õ���Ħ���ĵ��ӣ�����һĦ��Sn2+ת��Ϊ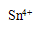 ��4��
��4��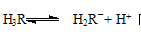 ����PHֵ������������Ũ�Ȼ��С��ƽ�������ƶ�������H3R��������١�H3R��H2R����HR2����R3����ͼ���б�ʾ�����߷ֱ�Ϊa��b��c��d���Դ�ͼ���Ͽ���B��ʽ��ߵ����Ժ���������H2R��
����PHֵ������������Ũ�Ȼ��С��ƽ�������ƶ�������H3R��������١�H3R��H2R����HR2����R3����ͼ���б�ʾ�����߷ֱ�Ϊa��b��c��d���Դ�ͼ���Ͽ���B��ʽ��ߵ����Ժ���������H2R��
���㣺�����ܽ�ƽ�⼰ƽ���ƶ������֪ʶ��

 53������ϵ�д�
53������ϵ�д�HAΪ������ǿ�ڴ����һԪ���ᣬ������������ȷ����
| A��0. 1 mol?L��1 HA��c(H+)= c(OH��)+ c(A��) |
| B��0. 1 mol?L��1 HA�� 0. 1 mol?L��1NaOH �������Һ�����ԣ�c(Na+)= c(A��) |
| C��0. 1 mol?L��1 NaA ��c(Na+)> c(OH��)>c(A��)> c(H+) |
| D��0. 1 mol?L��1 HA�м�������NaA���壬HA�ĵ��볣����С |
��14�֣����¶�t���£�ijBa(OH)2��ϡ��Һ��c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12�������Һ����μ���pH=b��NaHSO4����û����Һ�IJ���pH���±���ʾ��
| ��� | �������������/mL | �������Ƶ����/mL | ��Һ��pH |
| �� | 33.00 | 0.00 | 8 |
| �� | 33.00 | x | 7 |
| �� | 33.00 | 33.00 | 6 |
��2��b=____________��x =" ______mL" ��
��3����Ӧ�۵����ӷ���ʽΪ____________________________
��4�������¶��µ�Ba(OH)2��Һȡ��1mL����ˮϡ����1L����ϡ�ͺ���Һ��
c(Ba2+)�sc(OH��)= ��
��5�� ��NaHSO4��ͬ�� NaHSO3��NaHCO3ҲΪ��ʽ�Ρ���֪NaHSO3��Һ�����ԣ�NaHCO3��Һ�ʼ��ԡ�����Ũ�Ⱦ�Ϊ0.1mol/L��NaHSO3��Һ��NaHCO3��Һ����Һ�и����ӵ����ʵ���Ũ�ȴ������й�ϵ(R��ʾS��C)�����п�����ȷ����___________������ȷ�𰸵ı�ţ���
�� ��A��c(
 )��c(
)��c( )��c(
)��c( )��c(
)��c( )��c(
)��c( )
)����B��c(
 )��c(
)��c( )��c(
)��c( )��2c(
)��2c( )��c(
)��c( )
)����C��c(
 )��c(
)��c( )��c(
)��c( )��c(
)��c( )
)����D������Һ��c(
 )��c(
)��c( )��c(
)��c( )�ֱ����
)�ֱ����  ��Ҫ��С
��Ҫ��С