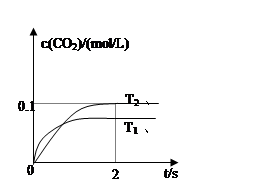��Ŀ����
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����±���JԪ��������ϼ۵ľ���ֵ����ԭ���������������
| | | J | | |
| | | | | R |
M����̬ԭ�����ʧȥ1~ 4���������������������ܣ����±���ʾ��
| | I1 | I2 | I3 | I4 | ���� |
| ������(kJ/mol) | 578 | 1817 | 2745 | 11578 | ���� |
��1��M�ĵ����Ų�ʽΪ________��Ԫ��T�����ڱ��е�λ��Ϊ________��
��2��J�������γɶ��ֻ�������з��ӳ�ֱ���͵ģ�����Է���������С�����ʵĽṹʽΪ________��
��3��M��T�γɵĻ������ڳ�ʪ�Ŀ�����ð��������Ӧ�Ļ�ѧ����ʽΪ_________________��
��4����J��R�γɵ�Һ̬������JR2 0.2 mol��O2����ȫȼ�գ�����������̬�����298 Kʱ�ų�����215 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
��5����Դ�����ѳ�Ϊ�����ѧ�о����ȵ㡣������Ϊһ�������Դ�����������Ĵ������⣬C60������������ϡ���C60��ѧ���ֺϳ���Si60��N60�������й�˵����ȷ����_______������ţ���
a. C60��Si60��N60���������ͻ�����
b. C60��Si60��N60��Ϊͬ���칹��
c. ��֪N60�ṹ��C60���ƣ�����N-N����С��N��N����N60���ȶ�������N2
d. ��֪���ʯ��C��C����154pm��C60��C��C����145~140pm����C60�۵���ڽ��ʯ
��1��1s22s22p63s23p1 �������ڢ�A��
��2��H��C��C��H
��3��AlCl3��3H2O��Al(OH)3��3HCl��
��4��CS2(l)��3O2(g) =CO2(g)��2SO2(g) ��H="-1075" kJ/mol��2�֣�
��5��c��2�֣�
�������������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�˵��J����ͻ��ϼ�������ϼ۾���ֵ��ȣ���J����������Ϊ4������J��R��Ϊ������Ԫ�ؿ�֪JΪCԪ�أ���RӦΪSԪ�ء�
��1����M�ĵ���������ǰ�����������ʧȥ��������I1 ��I2 ��I3����I4 ���dz����ɴ˿����Ʋ�ǰ3��������ͬһ����㣬��4�������ڴ���㣬�ɴ˿��Ʋ�MΪAlԪ�أ�����������Ų�Ϊ1s22s22p63s23p1 ��Ԫ��T����R��ߵ�����Ԫ�أ�R��SԪ�أ���T��ClԪ�أ�λ��Ϊ�������ڢ�A�塣
��2��JΪCԪ�أ����Ժ������γɶ��ֻ�����Ϊ�л���������г�ֱ���͵ĺ���C��C��Ȳ�࣬��Է�����С��Ȳ��Ϊ��Ȳ����ṹʽΪH��C��C��H��
��3��M��T�γɵĻ�����ΪAlCl3����һ��ǿ�������Σ��ڳ�ʪ����������ˮ������ӷ���HCl��Ȼ���γ����������Է�Ӧ����ʽΪAlCl3��3H2O=Al(OH)3��3HCl����
��4��J��R�γɵ�Һ̬������JR2 ΪCS2������ȫȼ������CO2��SO2��0.2mol�ų�����215kJ������1molʱȼ�շų�����1075 kJ�������Ȼ�ѧ����ΪCS2(l)��3O2(g) =CO2(g)��2SO2(g) ��H="-1075" kJ/mol��
��5��C60��Si60��N60���ǵ��ʣ�aѡ�����C60��Si60��N60��ɵ�Ԫ�ز�ͬ��������ͬ���칹�壬b���������N-N����С��N��N��N60�ṹ��C60���ƣ����γɵķ����ж��ǵ�������N60���ȶ�������N2 ��c��ȷ��d������Ȼ���ʯ��C��C����Ҫ����C60��C��C���������ǽ��ʯ�ǿռ���״�ṹ�dz��ȶ�����C60������������ģ�ͽṹ�����ȶ������Խ��ʯ�۵����C60�۵㣬d����ѡc��
���㣺���⿼�����Ԫ�����ڱ��������ɵ�֪ʶ��

A��B��C��D��E��F��G��H��Ԫ�����ڱ�ǰ�����ڳ���Ԫ�أ���ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| A | ԭ�Ӻ�����6�ֲ�ͬ�˶�״̬�ĵ��� |
| C | ��̬ԭ����s����������p����������� |
| D | ԭ�Ӱ뾶��ͬ����Ԫ������� |
| E | ��̬ԭ�����������Ų�ʽΪ3s23p1 |
| F | ��̬ԭ�ӵ������p������������ӵ������������������ӵ����������෴ |
| G | ��̬ԭ�Ӻ�����7���ܼ���������ߵ��ܼ�����6������ |
| H | ���ҹ�ʹ������ĺϽ��е�����ҪԪ�� |
���û�ѧ������գ�
��1��AԪ��λ��Ԫ�����ڱ��������������������壬BԪ�غ�CԪ�صĵ�һ�����ܱȽϣ��ϴ������������CԪ�غ�FԪ�صĵ縺�ԱȽϣ���С������������
��2��BԪ���������к�����ḻ��Ԫ���γɵ��������ķ��ӹ���Ϊ������ ��
BԪ�����γɵĵ��ʷ����ЦҼ���м���Ŀ֮��Ϊ������ ��
��3��GԪ�صĵͼ������ӵ����ӽṹʾ��ͼ��_____________��FԪ��ԭ�ӵļ۵��ӹ��͵ĵ����Ų�ͼ�������ʾʽ����__________________��HԪ�صĻ�̬ԭ�Ӻ�������Ų�ʽ��������������������
��4��G�ĸ������ӵ���Һ��H���ʷ�Ӧ�����ӷ���ʽΪ���������������������� ��
��EԪ�سɶԽǹ�ϵ��ijԪ�ص�����������ˮ����������ԣ�д��������������DԪ�ص�����������ˮ���ﷴӦ�Ļ�ѧ����ʽΪ�������������������� ��
 2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��