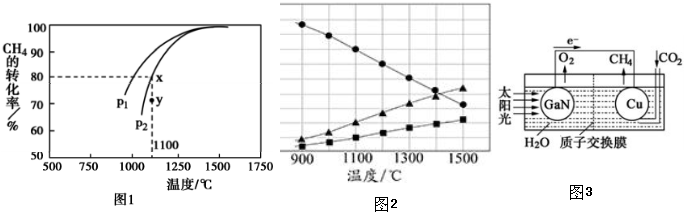
��Ŀ����
11�������ѡ���ж�Ӧ��ʵ�����������ͽ��۾���ȷ���ǣ�������| ѡ�� | ʵ����� | �� �� | �� �� |
| A | ���Թ��е�������C2H5X��NaOH��Һ�������ȡ����÷ֲ��ȡˮ���ϡ�����ữ���ٵμ�����AgNO3��Һ | ����ɫ���� | ֤��±�����к�����Ԫ�� |
| B | ��պ��Ũ��ˮ�IJ���������ij����Ũ��Һ���Լ�ƿ�� | �д������� | ������һ��Ϊ���� |
| C | �ڴ��Թ������μ��������������������ᡢ����Ũ���ᣬ��Ϻ����ϴ������ܵ��Թ���������ʯ�����Ϸ������м�����120������ | ��ӦҺ���ڣ���ȴ���ã��ϲ���״��Һ����ˮ����ζ | ����״Һ��Ϊ���ᶡ�� |
| D | �ڼ������շ���������ʯ��ʯ�м���Ũ���ᣬ������������ֱ��ͨ�뱽������Һ�� | ��������Һ�������� | ���ԣ����̼����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A��±�����ڼ�����Һ��ˮ�����������ӣ���ϡ�����ữ��AgNO3��Һ����Ӧ���ɵ���ɫ��AgBr��
B��Ũ��ˮ����лӷ��Ե�Ũ�ᷴӦ���ɹ��壻
C�������붡����Ӧ�������ᶡ����
D��ǿ���ܺ������η�Ӧ�������ᣬ������лӷ��ԣ�����Ҳ�ܺͱ����Ʒ�Ӧ���ɱ��ӣ�
��� �⣺A��±�����ڼ�����Һ��ˮ�����������ӣ���������������������£��μ�AgNO3��Һ����Ӧ����AgBr���۲쵽���ֵ���ɫ������˵��±�����к�����Ԫ�أ���A��ȷ��
B��Ũ��ˮ����лӷ��Ե�Ũ�ᷴӦ���ɹ��壬�������֪����ΪŨ���ᡢŨ����ȣ���B����
C�������붡����Ũ������·�Ӧ�������ᶡ������C��ȷ��
D��ǿ���ܺ������η�Ӧ�������ᣬ������лӷ��ԣ�������ȡ�Ķ�����̼�к��д��ᣬ����Ҳ�ܺͱ����Ʒ�Ӧ���ɱ��ӣ����Բ��ܾݴ��ж����ԣ����̼����ӣ���D����
��ѡAC��
���� ���⿼�黯ѧʵ�鷽�����ۣ�Ϊ�߿���Ƶ�㣬���ؿ������ԭ�������Ӽ����֪ʶ�㣬��ȷԪ�ػ�����֪ʶ���������ʼ��ɽ���״�ѡ����D��ע���ų�����ĸ��ţ�Ϊ�״��㣮

��ϰ��ϵ�д�
 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
�����Ŀ
1����Ԫ�ؿɱ�ʾΪ 83Bi����Ԫ�����ڱ������ԭ��������ʾΪ209.0������˵����ȷ���ǣ�������
| A�� | BiԪ�ص���������209 | |
| B�� | BiԪ�����������Ļ�ѧʽΪBi2O3 | |
| C�� | Biԭ������������ˮ�������Ա������Ҫǿ | |
| D�� | BiԪ��λ��Ԫ�����ڱ��������ڵڢ�A�� |
6��Һ̬���пɵ������������NH2-��NH4+������˵����ȷ���ǣ�������
| A�� | NH3�������ӻ����� | |
| B�� | �����£�Һ���ĵ���ƽ�ⳣ��Ϊ10-14 | |
| C�� | Һ̬���백ˮ�������ͬ | |
| D�� | Һ���е�������ͬ�ĵ����� |
3������W��Z֮���з�Ӧ��4W+O2+2H2O=4Z������˵����ȷ���ǣ�������
| A�� | ����W��Z������Ԫ�ؿ�����ͬҲ���ܲ���ͬ | |
| B�� | ��W��һ�����壬�����ɰ����Ĵ�����ֱ������ | |
| C�� | ����W��Z�����е�ij��Ԫ�أ������ǵؿ��л�����к�����ߵ� | |
| D�� | ÿת��1mol���ӣ���������5.6L |
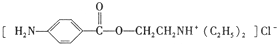 ����һ�־ֲ����������������ýϿ졢��ǿ�����Խϵͣ���ϳ�·�����£�
����һ�־ֲ����������������ýϿ졢��ǿ�����Խϵͣ���ϳ�·�����£�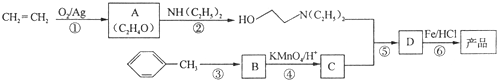
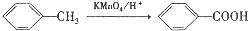
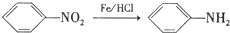
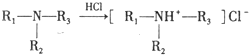
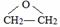 ��
��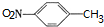 ��C�к��������ŵ�����Ϊ�������Ȼ���
��C�к��������ŵ�����Ϊ�������Ȼ���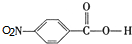 +HOCH2CH2N��C2H5��2$\stackrel{һ��������}{��}$
+HOCH2CH2N��C2H5��2$\stackrel{һ��������}{��}$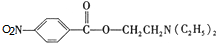 +H2O��
+H2O��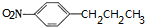 ����дһ�֣���
����дһ�֣���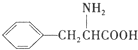 ���������������İ�����֮һ��д���䷢�����۷�Ӧ�Ļ�ѧ����ʽ
���������������İ�����֮һ��д���䷢�����۷�Ӧ�Ļ�ѧ����ʽ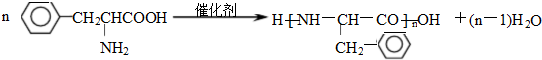 ��
��