��Ŀ����
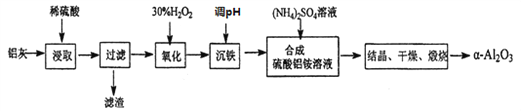
����Ŀ��.ij��ȤС�����10.0 g�������ޣ���90%��Al��������������Fe��Mg�����ʣ��Ʊ�����[KAl(SO4)2��12H2O]��ʵ��������ͼ��

��1���Լ���Ӧѡ��________������ĸ����
a.���� b.H2SO4��Һ c.��ˮ d.NaOH��Һ
��2���������ܽ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ__________________________��
��3������ҺC�еõ�������ʵ�����Ϊ________��________�����ˣ�����ͼ��ʾװ�ý��иò��������е�һ����Ҫ������______________��
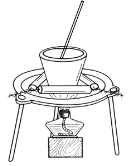
���𰸡�d 2Al��2NaOH��2H2O=2NaAlO2��3H2�� ����Ũ�� ��ȴ�ᾧ ��������ʹ������
��������
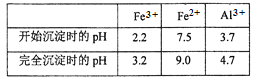
Al����ǿ�Ӧ����Fe��Mg����Ӧ�����Լ���ΪNaOH��Һ����ҺAΪƫ�����ƺ�����δ��Ӧ��NaOH������AΪFe��Mg���壻��ƫ��������Һ��ͨ������Ķ�����̼��������������������̼��������Һ�������BΪ������������ҺBΪ̼��������Һ�����������������������صĻ��Һ������ҺCΪ����������Һ��
(1)������֪���Լ���ΪNaOH��Һ����Ϊd��
(2)�������е�Al��NaOH��Ӧ����ƫ�����ƺ�����������ʽΪ2Al��2NaOH��2H2O=2NaAlO2��3H2����
(3)��ҺCΪ����������Һ���ɲ�������Ũ�������½ᾧ�����ˡ�ϴ�ӵķ����õ�������������Һʱ��ʹ��������ʹ��������

����Ŀ�������й�ʵ��IJ�����ȷ����
ʵ��Ŀ�� | ʵ����� | |
A | ����Ũ��Ϊ0.010 | ��������ƽ��ȡKMnO4����0.158 g������100 mL����ƿ�У���ˮ�ܽⲢϡ�����̶� |
B | Ũ������MnO2��Ӧ�Ʊ�����Cl2 | ���������ͨ��Ũ���ᣬ��ͨ������ʳ��ˮ |
C | ����ϡ���� | �Ƚ�Ũ��������ձ��У���������ˮ |
D | ��ˮ���ռ�KMnO4�ֽ������O2 | ���Ƴ����ܣ���Ϩ��ƾ��� |
A.AB.BC.CD.D
����Ŀ�����ڷ��õ�FeSO4��Һ�ױ����������ʣ�ij��ȤС�����������ʵ�飺
(1)���ʵ�����FeSO4��Һ�ı��ʳ̶�
ʵ�鷽�� | ʵ������ | ʵ����� | |
����1 | ȡ�����Һ���Թ��У������еμ�KSCN��Һ | ________ | FeSO4��Һ���ֱ��� |
����2 | _____ | _________ | |
�� ������������������
�� ��Ҫʹ���ֱ��ʵ�FeSO4��ԭ��������__________��(д���ӷ�Ӧ����ʽ)
(2)�������ֱ�����FeSO4��Һ�Ʊ�Fe2O3
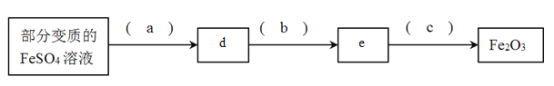
����д���и��գ�a._____b._______c.________d._____e.______
����100mL�ñ�����Һ�Ƶ�1.6gFe2O3�������ǰFeSO4��Һ��Ũ��Ϊ__________��
(3)FeSO4��������������ʹ��ʱ������ά����Cͬ����ͬѧ�ײ²�ά����C�ɽ�Fe3+ת��ΪFe2+���������������ա�Ϊ����֤��һ���룬���������ʵ�飺
ʵ�鷽�� | ʵ������ |
ȡ���� Fe2(SO4)3��Һ���Թ��У�����ά����CƬ�����ܽ�μ����Ը��������Һ�� | ��ɫ��ȥ |
������ʵ���ܷ�ó���ά����C�ɽ�Fe3+ת��ΪFe2+���Ľ��ۣ���˵������_______��