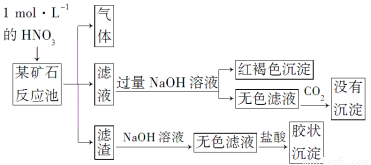
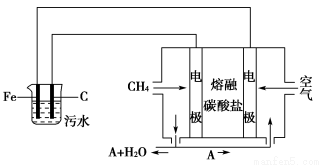
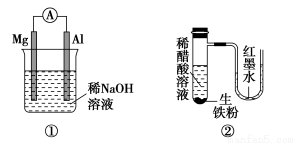
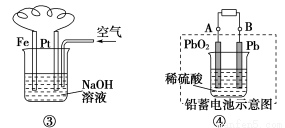
��Ŀ����
��15.6 g Na2O2��5.4 g Alͬʱ����һ������ˮ�У���ַ�Ӧ��õ�200 mL��Һ���������Һ�л���ͨ���״���µ�HCl����6.72 L������Ӧ��������Һ��������ֲ��䣬������˵����ȷ����(����)
A����״���£���Ӧ�����еõ�7.84 L������
B�����յõ�����Һ��c(Na��)��c(Cl��)��c(OH��)
C�����յõ�7.8 g�ij���
D�����յõ�����Һ��c(Na��)��1.5 mol��L��1
��C
����������2Na2O2��2H2O=4NaOH��O2��
0��2 mol���� 0.4 mol��0.1 mol
��2Al��2NaOH��2H2O=2NaAlO2��3H2��
0��2 mol 0.4 mol���� 0.2 mol��0.3 mol
��������������ʽ��֪�����ɵ��������Ϊ��(0.1 mol��0.3 mol)��0.4 mol��Ϊ8.96 L����A����n(Na��)��0.4 mol����c(Na��)��0.4 mol/0.2 L��2 mol��L��1����D����
��Ӧ��ʣ��0.2 mol NaOH����0.3 mol HCl�����䷴Ӧ��ʣ���0.1 mol HCl����NaAlO2��Ӧ������0.1 mol Al(OH)3����������Ϊ7.8 g����C��ȷ�����յõ�����0.1 mol NaCl��0.1 mol NaAlO2�Ļ����Һ���ݵ���غ��У�c(Na��)��c(H��)��c(Cl��)��c(OH��)��c(AlO2��)������c(H��)��c(AlO)����B����

 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�ijС��ͬѧ��̼��Ϊ�缫���CuCl2��Һʱ����������̼���ϳ����к�ɫ���������⣬����������ɫ����������Ϊ̽������̼���ϵIJ��ͬѧ���Ķ����ϲ���������¹��̣�
��.�й����ϣ�ͭ�Ļ�������ɫ��������
���� | ��ɫ������ | ���� | ��ɫ������ |
������ͭCu(OH)2 | ��ɫ���岻����ˮ | ����ͭ(CuSO4) | ��Һ����ɫ |
������ͭ(Cu2O) | ��ɫ���岻����ˮ | �Ȼ�ͭ(CuCl2) | Ũ��Һ����ɫ��ϡ��Һ����ɫ |
�Ȼ���ͭ(CuCl) | ��ɫ���岻����ˮ | ��ʽ�Ȼ�ͭ | ��ɫ���岻����ˮ |
��.̽��ʵ�飺
(1)�������
����ɫ����һ����ͭ����������Cu2O��
����ɫ����Ϊͭ�Ļ�����仯ѧʽ����Ϊ__________________________��
(2)ʵ����֤
ȡ���CuCl2��Һ�������̼����ϴ�ӡ������������װ�ý���ʵ�飬��֤�������

��ʵ��ǰ�����װ��A�����Եķ�����_______________________________��
��ʵ��ʱ����װ�ô������ҵ�����˳��ΪA��________��________��B��________��________��
(3)�۲����ó�����
ʵ�������̼���ϵİ�ɫ���ʱ�Ϊ��ɫ��F�����ʲ���ɫ��D�г��ְ�ɫ����������������̼���ϵĺ�ɫ�����Ƿ���Cu2O________(����������������)��������________________________________________________________��
��װ��________(����ͼ��װ�ñ��)��________������˵������������еİ�ɫ����һ�����ڣ�
��д��װ��B�з�����Ӧ�Ļ�ѧ����ʽ___________________________ _��
(4)��������
�����CuCl2��Һ��������Ϸ����ķ�ӦΪ��______________________
��_______________________________________________________________��
��ʵ������У���װ��B�еĿ���û���ž��Ϳ�ʼ���ȣ����ܶ�ʵ����ɵ�Ӱ����__________________________________________________________��