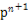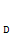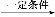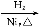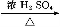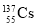ΧβΡΩΡΎ»ί
Α± «ΒΣ―≠ΜΖΙΐ≥Χ÷–ΒΡ÷Ί“ΣΈο÷ ,Α±ΒΡΚœ≥… «ΡΩ«ΑΤ’±ι Ι”ΟΒΡ»ΥΙΛΙΧΒΣΖΫΖ®ΓΘ
(1)ΗυΨίΆΦ1ΧαΙ©ΒΡ–≈œΔ,–¥≥ωΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ:ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ,‘ΎΆΦ1÷–«ζœΏΓΓΓΓΓΓΓΓΓΓΓΓΓΓ(ΧνΓΑaΓ±ΜρΓΑbΓ±)±μ ΨΦ”»κΧζ¥ΞΟΫΒΡΡήΝΩ±δΜ·«ζœΏΓΘ
(2)‘ΎΚψ»ί»ίΤς÷–,œ¬Ν–Οη ω÷–ΡήΥΒΟς…œ ωΖ¥”Π“―¥οΤΫΚβΒΡ «ΓΓΓΓΓΓΓΓΓΘ
A.3v(H2)’ΐ=2v(NH3)Ρφ
B.ΒΞΈΜ ±ΦδΡΎ…ζ≥…n mol N2ΒΡΆ§ ±…ζ≥…2n mol NH3
C.ΜλΚœΤχΧεΒΡΟήΕ»≤Μ‘ΌΗΡ±δ
D.»ίΤςΡΎ―Ι«Ω≤ΜΥφ ±ΦδΒΡ±δΜ·Εχ±δΜ·
(3)“ΜΕ®Έ¬Ε»œ¬,œρ2 LΟή±’»ίΤς÷–≥δ»κ1 mol N2ΚΆ3 mol H2,±Θ≥÷ΧεΜΐ≤Μ±δ,0.5 minΚσ¥οΒΫΤΫΚβ,≤βΒΟ»ίΤς÷–”–0.4 mol NH3,‘ρΤΫΨυΖ¥”ΠΥΌ¬ v(N2)=ΓΓΓΓΓΓΓΓΓΓΓΓ,ΗΟΈ¬Ε»œ¬ΒΡΤΫΚβ≥Θ ΐK=ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ»τ…ΐΗΏΈ¬Ε»,K÷Β±δΜ·ΓΓΓΓΓΓΓΓ(ΧνΓΑ‘ω¥σΓ±ΓΔΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)ΓΘ
(4)ΈΣΝΥ―Α’“Κœ≥…NH3ΒΡΈ¬Ε»ΚΆ―Ι«ΩΒΡ “ΥΧθΦΰ,Ρ≥Ά§―ß…ηΦΤΝΥ»ΐΉι Β―ι,≤ΩΖ÷ Β―ιΧθΦΰ“―Ψ≠Χν‘Ύœ¬Οφ Β―ι…ηΦΤ±μ÷–ΓΘ
Β―ι±ύΚ≈ | T(Γφ) | n(N2)/n(H2) | p(MPa) |
ΔΓ | 450 | 1/3 | 1 |
ΔΔ | ΓΓ | ΓΓ | 10 |
ΔΘ | 480 | ΓΓ | 10 |
A.«κ‘Ύ…œ±μΩ’Ηώ÷–Χν»κ Θ”ύΒΡ Β―ιΧθΦΰ ΐΨίΓΘ
B.ΗυΨίΖ¥”ΠN2(g)+3H2(g) 2NH3(g)ΒΡΧΊΒψ,‘ΎΗχ≥ωΒΡΉχ±ξΆΦ2÷–,Μ≠≥ωΤδ‘Ύ1 MPaΚΆ10 MPaΧθΦΰœ¬H2ΒΡΉΣΜ·¬ ΥφΈ¬Ε»±δΜ·ΒΡ«ς Τ«ζœΏ Ψ“βΆΦ,≤Δ±ξΟςΗςΧθ«ζœΏΒΡ―Ι«ΩΓΘ
2NH3(g)ΒΡΧΊΒψ,‘ΎΗχ≥ωΒΡΉχ±ξΆΦ2÷–,Μ≠≥ωΤδ‘Ύ1 MPaΚΆ10 MPaΧθΦΰœ¬H2ΒΡΉΣΜ·¬ ΥφΈ¬Ε»±δΜ·ΒΡ«ς Τ«ζœΏ Ψ“βΆΦ,≤Δ±ξΟςΗςΧθ«ζœΏΒΡ―Ι«ΩΓΘ
(1)N2(g)+3H2(g) 2NH3(g)ΠΛH=-92 kJΓΛmol-1ΓΓb
2NH3(g)ΠΛH=-92 kJΓΛmol-1ΓΓb
(2)BDΓΓ(3)0.2 molΓΛL-1ΓΛmin-1ΓΓ0.058ΓΓΦθ–Γ
(4)A.ΔΔ.450ΓΓ1/3ΓΓΔΘ.1/3
B.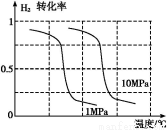
ΓΨΫβΈωΓΩ(1)”…ΆΦΩ…Ω¥≥ωΗΟΖ¥”ΠΈΣΖ≈»»Ζ¥”Π,ΠΛH=-(600 kJΓΛmol-1-508 kJΓΛmol-1)=-92 kJΓΛmol-1,Ι »»Μ·―ßΖΫ≥Χ ΫΈΣN2(g)+3H2(g) 2NH3(g)ΓΓΠΛH=-92 kJΓΛmol-1,Φ”»κ¥ΏΜ·ΦΝΡήΫΒΒΆΖ¥”ΠΒΡΜνΜ·Ρή,Ι b«ζœΏ±μ ΨΦ”»κΝΥ¥ΏΜ·ΦΝΓΘ
2NH3(g)ΓΓΠΛH=-92 kJΓΛmol-1,Φ”»κ¥ΏΜ·ΦΝΡήΫΒΒΆΖ¥”ΠΒΡΜνΜ·Ρή,Ι b«ζœΏ±μ ΨΦ”»κΝΥ¥ΏΜ·ΦΝΓΘ
(2)Β±2v(H2)’ΐ=3v(NH3)Ρφ ±Ζ¥”Π¥οΤΫΚβ,A≤ΜΡήΥΒΟς;“ρ «Κψ»ί»ίΤς«“Ζ¥”ΠΈοΚΆ≤ζΈοΕΦ «ΤχΧε,Ι ΟήΕ» Φ÷’≤Μ±δ,C≤ΜΡήΥΒΟςΓΘ
(3)Ν–»ΐΕΈ Ϋ»γœ¬:
ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓN2(g)+3H2(g) 2NH3(g)
2NH3(g)
Τπ Φ(molΓΛL-1) 0.5 1.5 0
ΉΣΜ·(molΓΛL-1) 0.10.3 0.2
ΤΫΚβ(molΓΛL-1) 0.4 1.2 0.2
v(N2)=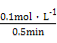 =0.2 molΓΛL-1ΓΛmin-1,K=
=0.2 molΓΛL-1ΓΛmin-1,K=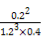 Γ÷0.058,ΗΟΖ¥”Π «Ζ≈»»Ζ¥”Π,…ΐΗΏΈ¬Ε»ΤΫΚβΡφœρ“ΤΕ·,KΦθ–ΓΓΘ
Γ÷0.058,ΗΟΖ¥”Π «Ζ≈»»Ζ¥”Π,…ΐΗΏΈ¬Ε»ΤΫΚβΡφœρ“ΤΕ·,KΦθ–ΓΓΘ
(4)A.“Σ―Α’“ “ΥΧθΦΰ,”Π ΙΤδ÷–ΝΫ’ΏœύΆ§»Ξ±»ΫœΒΎ»ΐ’Ώ,±ΨΧβ÷Μ «¥”Έ¬Ε»ΚΆ―Ι«ΩΝΫΖΫΟφΩΦ¬«,Ι  ”ΠΚψΒ»”Ύ
”ΠΚψΒ»”Ύ ,‘ρΔΓ”κΔΔ”ΠΈ¬Ε»œύΆ§,ΔΔ”κΔΘ”Π―Ι«ΩœύΆ§ΓΘ
,‘ρΔΓ”κΔΔ”ΠΈ¬Ε»œύΆ§,ΔΔ”κΔΘ”Π―Ι«ΩœύΆ§ΓΘ
B.Κœ≥…Α± «“ΜΗωΤχΧεΧεΜΐΦθ–ΓΒΡΖ≈»»Ζ¥”Π,ΥφΉ≈Έ¬Ε»ΒΡ…ΐΗΏ,H2ΒΡΉΣΜ·¬ Φθ–Γ,―Ι«Ω‘Ϋ¥σ,H2ΒΡΉΣΜ·¬ ‘Ϋ¥σ,Ι ΥφΈ¬Ε»…ΐΗΏ«ζœΏ”–Φθ–Γ«ς Τ,«“10 MPa«ζœΏ”Π‘Ύ1 MPa«ζœΏ÷°…œΓΘ