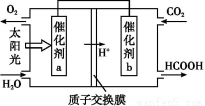
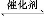جâؤ؟ؤعبف
دآ±يخھضـئع±يµؤز»²؟·ض,ئنضذµؤ±à؛إ´ْ±ي¶شس¦µؤشھثط،£
اë»ط´ًدآءذختجâ:
(1)±يضذتôسعdاّµؤشھثطتا،،،،،،،،(جî±à؛إ)،£
(2)شھثط¢قذخ³ةµؤ×î¸ك¼غ؛¬رُثل¸ùµؤء¢جه¹¹ذحتا،،،،،،،،,ئنضذذؤش×سµؤشس»¯¹ىµہہàذحتا،،،،،،،،،£
(3)شھثط¢عµؤز»ضضا⻯خïتاضطزھµؤ»¯¹¤شءد,³£°ر¸أا⻯خïµؤ²ْء؟×÷خھ؛âء؟ت¯سح»¯¹¤·¢ص¹ث®ئ½µؤ±êض¾،£سذ¹ط¸أا⻯خï·ض×سµؤثµ·¨صب·µؤتا،،،،،،،،،£
A.·ض×سضذ؛¬سذاâ¼ü
B.تôسع·ا¼«ذش·ض×س
C.؛¬سذ4¸ِ¦ز¼ü؛ح1¸ِ¦ذ¼ü
D.¸أا⻯خï·ض×سضذ,¢عش×س²ةسأsp2شس»¯
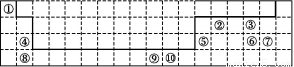
(4)ؤ³شھثطµؤجطص÷µç×سإإ²¼ت½خھnsnn ,¸أشھثطش×سµؤ؛ثحâ×îحâ²مµç×سµؤ¹آµç×س¶شتخھ،،،،،،،،;¸أشھثطسëشھثط¢ظذخ³ةµؤ·ض×سX¹¹ذخخھ،،،،،،،،;Xشع¢ظسë¢غذخ³ةµؤ·ض×سYضذµؤبـ½â¶ب؛ـ´َ,ئنض÷زھشزٍتا ،£
,¸أشھثطش×سµؤ؛ثحâ×îحâ²مµç×سµؤ¹آµç×س¶شتخھ،،،،،،،،;¸أشھثطسëشھثط¢ظذخ³ةµؤ·ض×سX¹¹ذخخھ،،،،،،،،;Xشع¢ظسë¢غذخ³ةµؤ·ض×سYضذµؤبـ½â¶ب؛ـ´َ,ئنض÷زھشزٍتا ،£
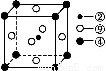
(5)؟ئر§·¢دض,¢ع،¢¢ـ،¢¢لبضضشھثطµؤش×سذخ³ةµؤ¾§جه¾كسذ³¬µ¼ذش,ئن¾§°ûµؤ½ل¹¹جطµمبçح¼(ح¼ضذ¢ع،¢¢ـ،¢¢ل·ض±ًخ»سع¾§°ûµؤجهذؤ،¢¶¥µم،¢أوذؤ),شٍ¸أ»¯؛دخïµؤ»¯ر§ت½خھ،،،،،،،،(سأ¶شس¦µؤشھثط·û؛إ±يت¾)،£
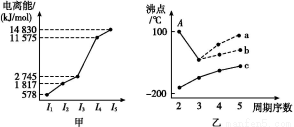
(1)¢ل،،(2)صثؤأوجهذخ،،sp3،،(3)BD
(4)3،،ب½ا׶ذخ،،شعNH3·ض×س؛حث®·ض×س¼نسذاâ¼ü×÷سأ
(5)MgNi3C
،¾½âخِ،؟(1)¸ù¾فشھثطضـئع±ي½ل¹¹؟ة»ط´ً،£(2)¢ق¶شس¦µؤ؛¬رُثل¸ùتاS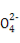 ,¸ù¾فµç×س»¥³âہيآغ,؟ةإذ¶دS
,¸ù¾فµç×س»¥³âہيآغ,؟ةإذ¶دS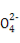 خھصثؤأوجه½ل¹¹,ضذذؤش×س²ةسأsp3شس»¯،£(3)شھثط¢عخھج¼شھثط,¸ù¾ف¸أشھثطµؤا⻯خïµطخ»؛حسأح¾؟ةإذ¶د¸أا⻯خïخھCH2
خھصثؤأوجه½ل¹¹,ضذذؤش×س²ةسأsp3شس»¯،£(3)شھثط¢عخھج¼شھثط,¸ù¾ف¸أشھثطµؤا⻯خïµطخ»؛حسأح¾؟ةإذ¶د¸أا⻯خïخھCH2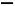 CH2(ززد©),¸أ·ض×سضذC،ھH¼üخھ¦ز¼ü,C
CH2(ززد©),¸أ·ض×سضذC،ھH¼üخھ¦ز¼ü,C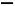 Cث«¼üضذسذز»¸ِ¦ز¼ü؛حز»¸ِ¦ذ¼ü,ئنضذCش×س²ةسأsp2شس»¯،£¸أ·ض×س¸ك¶ب¶ش³ئ,تôسع·ا¼«ذش·ض×س،£ر،دîBDصب·،£(4)nsnn
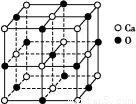
Cث«¼üضذسذز»¸ِ¦ز¼ü؛حز»¸ِ¦ذ¼ü,ئنضذCش×س²ةسأsp2شس»¯،£¸أ·ض×س¸ك¶ب¶ش³ئ,تôسع·ا¼«ذش·ض×س،£ر،دîBDصب·،£(4)nsnn خھpاّشھثط,ش×سإإ²¼µ½p¹ىµہت±,s¹ىµہس¦جî³ن2¸ِµç×س,¹تn=2,¸أشھثطµؤ×îحâ²مµç×سإإ²¼خھ2s22p3،£¸أشھثطخھNشھثط,ئنضذ2s¹ىµہضذµؤµç×سخھ¹آµç×س¶ش،£¢ظخھHشھثط,NسëHذخ³ةNH3,سةسعNش×س´وشع¹آµç×س¶ش,¹تNH3·ض×سخھب½ا׶ذخ،£¢ظ؛ح¢غذخ³ةµؤ»¯؛دخïخھH2O,NH3؛حH2Oض®¼ن؟ةذخ³ةاâ¼ü,¹تNH3ز×بـسعث®،£(5)¢ع،¢¢ـ،¢¢ل¶شس¦µؤشھثطخھC،¢Mg؛حNiشھثط،£Cش×س´¦سعجهذؤ,¼´ئ½¾ùأ؟¸ِ¾§°û؛¬سذ1¸ِج¼ش×س;Mgش×س´¦سع¶¥µم,ئ½¾ùMgش×س¸ِتخھ8،ء1/8=1;Niش×سخ»سعأوذؤ,Niش×س¸ِتخھ6،ء1/2=3,¸أ»¯؛دخﻯر§ت½خھMgNi3C،£
خھpاّشھثط,ش×سإإ²¼µ½p¹ىµہت±,s¹ىµہس¦جî³ن2¸ِµç×س,¹تn=2,¸أشھثطµؤ×îحâ²مµç×سإإ²¼خھ2s22p3،£¸أشھثطخھNشھثط,ئنضذ2s¹ىµہضذµؤµç×سخھ¹آµç×س¶ش،£¢ظخھHشھثط,NسëHذخ³ةNH3,سةسعNش×س´وشع¹آµç×س¶ش,¹تNH3·ض×سخھب½ا׶ذخ،£¢ظ؛ح¢غذخ³ةµؤ»¯؛دخïخھH2O,NH3؛حH2Oض®¼ن؟ةذخ³ةاâ¼ü,¹تNH3ز×بـسعث®،£(5)¢ع،¢¢ـ،¢¢ل¶شس¦µؤشھثطخھC،¢Mg؛حNiشھثط،£Cش×س´¦سعجهذؤ,¼´ئ½¾ùأ؟¸ِ¾§°û؛¬سذ1¸ِج¼ش×س;Mgش×س´¦سع¶¥µم,ئ½¾ùMgش×س¸ِتخھ8،ء1/8=1;Niش×سخ»سعأوذؤ,Niش×س¸ِتخھ6،ء1/2=3,¸أ»¯؛دخﻯر§ت½خھMgNi3C،£

 شؤ¶ء؟ى³µدµءذ´ً°¸
شؤ¶ء؟ى³µدµءذ´ً°¸ؤ³رذ¾؟ذ،×齫ز»إْ·دئْµؤدكآ·°ه¾إ¨دُثل؛حد،ءٍثل´¦ہي؛َµأµ½ز»»ى؛دبـز؛,ئنضذ؛¬سذCu2+،¢Fe2+،¢Fe3+،¢Al3+µب½ًتôہë×س,²¢ةè¼ئءثزشدآء½ضضء÷³جزش·ض±ًضئب،CuSO4،¤5H2O¾§جه؛حAlCl3
بـز؛:
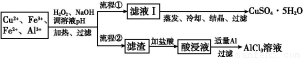
زرضھ:دà¹ط½ًتôہë×س؟ھت¼³ءµيضءحêب«³ءµيت±µؤpH·¶خ§خھ:
ہë×س | Fe3+ | Fe2+ | Al3+ | Cu2+ |
pH·¶خ§ | 2.2،«3.2 | 5.5،«9.0 | 4.1،«5.0 | 5.3،«6.6 |
اë»ط´ًدآءذختجâ:
(1)¼سبëH2O2µؤ×÷سأتا،، ,سûت¹ضئب،µؤCuSO4،¤5H2O¾§جه½دخھ´؟¾»,pHضءةظس¦µ÷ضء،،،،،،،،،£
(2)ذ´³ِH2O2سëFe2+·´س¦µؤہë×س·½³جت½: ،£
(3)ء÷³ج¢عضذ¼سبëتتء؟Al·غئًµؤ×÷سأتا ،،،،،،،£
(4)¸ù¾فؤمثùر§µؤ»¯ر§ضھت¶,سةAlCl3بـز؛(²»جي¼سئنثû»¯ر§تش¼ء)ؤـ·ٌضئµأخقث®AlCl3،،،،،،،،(جî،°ؤـ،±»ٍ،°²»ؤـ،±),شزٍتا،، ،£
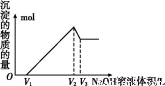
(5)ب،جه»خھV(L)µؤثل½ز؛,دٍئنضذµخ¼سa mol،¤L-1µؤNaOHبـز؛,ةْ³ة³ءµيµؤخïضتµؤء؟سëثù¼سµؤNaOHبـز؛µؤجه»(L)¹طدµبçح¼،£اëسأV1،¢V2،¢V3±يت¾ثùب،µؤثل½ز؛ضذn(Fe3+)،أn(Al3+)=،، ،£
°±تاµھر»·¹³جضذµؤضطزھخïضت,°±µؤ؛د³ةتاؤ؟ا°ئص±éت¹سأµؤبث¹¤¹جµھ·½·¨،£
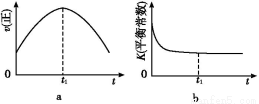
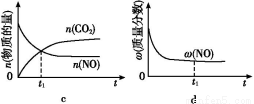
(1)¸ù¾فح¼1جل¹©µؤذإد¢,ذ´³ِ¸أ·´س¦µؤبب»¯ر§·½³جت½:،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،,شعح¼1ضذاْدك،،،،،،،،،،،،،،(جî،°a،±»ٍ،°b،±)±يت¾¼سبëجْ´¥أ½µؤؤـء؟±ن»¯اْدك،£
(2)شع؛مبفبفئ÷ضذ,دآءذأèتِضذؤـثµأ÷ةدتِ·´س¦زر´ïئ½؛âµؤتا،،،،،،،،،£
A.3v(H2)ص=2v(NH3)ؤو
B.µ¥خ»ت±¼نؤعةْ³ةn mol N2µؤح¬ت±ةْ³ة2n mol NH3
C.»ى؛دئّجهµؤأـ¶ب²»شظ¸ؤ±ن
D.بفئ÷ؤعر¹ا؟²»ثوت±¼نµؤ±ن»¯¶ّ±ن»¯
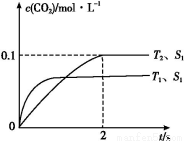
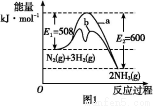
(3)ز»¶¨خآ¶بدآ,دٍ2 Lأـ±صبفئ÷ضذ³نبë1 mol N2؛ح3 mol H2,±£³ضجه»²»±ن,0.5 min؛َ´ïµ½ئ½؛â,²âµأبفئ÷ضذسذ0.4 mol NH3,شٍئ½¾ù·´س¦ثظآتv(N2)=،،،،،،،،،،،،,¸أخآ¶بدآµؤئ½؛â³£تK=،،،،،،،،،،،،،،،£بôة¸كخآ¶ب,Kضµ±ن»¯،،،،،،،،(جî،°شِ´َ،±،¢،°¼ُذ،،±»ٍ،°²»±ن،±)،£
(4)خھءثر°صز؛د³ةNH3µؤخآ¶ب؛حر¹ا؟µؤتتزثجُ¼,ؤ³ح¬ر§ةè¼ئءثب×éتµرé,²؟·ضتµرéجُ¼زر¾جîشعدآأوتµرéةè¼ئ±يضذ،£
تµرé±à؛إ | T(،و) | n(N2)/n(H2) | p(MPa) |
¢، | 450 | 1/3 | 1 |
¢¢ | ،، | ،، | 10 |
¢£ | 480 | ،، | 10 |
A.اëشعةد±ي؟ص¸ٌضذجîبëت£سàµؤتµرéجُ¼ت¾ف،£
B.¸ù¾ف·´س¦N2(g)+3H2(g) 2NH3(g)µؤجطµم,شع¸ّ³ِµؤ×ّ±êح¼2ضذ,»³ِئنشع1 MPa؛ح10 MPaجُ¼دآH2µؤ×ھ»¯آتثوخآ¶ب±ن»¯µؤا÷تئاْدكت¾زâح¼,²¢±êأ÷¸÷جُاْدكµؤر¹ا؟،£
2NH3(g)µؤجطµم,شع¸ّ³ِµؤ×ّ±êح¼2ضذ,»³ِئنشع1 MPa؛ح10 MPaجُ¼دآH2µؤ×ھ»¯آتثوخآ¶ب±ن»¯µؤا÷تئاْدكت¾زâح¼,²¢±êأ÷¸÷جُاْدكµؤر¹ا؟،£