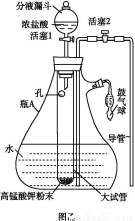
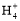ΧβΡΩΡΎ»ί
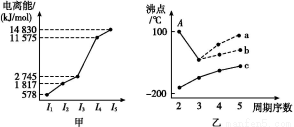
Δώ.Ϋπ τΡχΦΑΤδΜ·ΚœΈο‘ΎΚœΫπ≤ΡΝœ“‘ΦΑ¥ΏΜ·ΦΝΒ»ΖΫΟφ”Π”ΟΙψΖΚΓΘ
(1)ΜυΧ§Ρχ‘≠Ή”ΒΡΦέΒγΉ”(ΆβΈßΒγΉ”)≈≈≤Φ ΫΈΣΓΓ ΓΘ
(2)Ϋπ τΡχΡή”κCO–Έ≥…≈δΚœΈοNi(CO)4,–¥≥ω”κCOΜΞΈΣΒ»ΒγΉ”ΧεΒΡ“Μ÷÷Ζ÷Ή”ΚΆ“Μ÷÷άκΉ”ΒΡΜ·―ß ΫΓΓΓΓΓΓΓΓΓΔΓΓΓΓΓΓΓΓΓΘ
(3)ΚήΕύ≤Μ±ΞΚΆ”–ΜζΈο‘ΎNi¥ΏΜ·œ¬Ω…”κH2ΖΔ…ζΦ”≥…Ζ¥”ΠΓΘ
»γΔΌCH2 CH2ΓΔΔΎHCΓ‘CHΓΔΔέ
CH2ΓΔΔΎHCΓ‘CHΓΔΔέ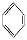 ΓΔΔήHCHO,Τδ÷–ΧΦ‘≠Ή”≤…»Γsp2‘”Μ·ΒΡΖ÷Ή””– (ΧνΈο÷ –ρΚ≈),HCHOΖ÷Ή”ΒΡΝΔΧεΫαΙΙΈΣΓΓΓΓΓΓΓΓ–ΈΓΘ
ΓΔΔήHCHO,Τδ÷–ΧΦ‘≠Ή”≤…»Γsp2‘”Μ·ΒΡΖ÷Ή””– (ΧνΈο÷ –ρΚ≈),HCHOΖ÷Ή”ΒΡΝΔΧεΫαΙΙΈΣΓΓΓΓΓΓΓΓ–ΈΓΘ
(4)Ni2+ΚΆFe2+ΒΡΑκΨΕΖ÷±πΈΣ69 pmΚΆ78 pm,‘ρ»έΒψNiOΓΓΓΓΓΓΓΓFeO(ΧνΓΑ<Γ±ΜρΓΑ>Γ±)ΓΘ
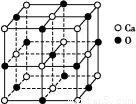
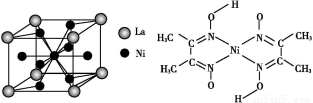
(5)Ϋπ τΡχ”κογ(La)–Έ≥…ΒΡΚœΫπ «“Μ÷÷ΝΦΚΟΒΡ¥Δ«β≤ΡΝœ,ΤδΨßΑϊΫαΙΙ Ψ“βΆΦ»γΉσœ¬ΆΦΥυ ΨΓΘΗΟΚœΫπΒΡΜ·―ß ΫΈΣΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ
(6)ΕΓΕΰΆΣκΩ≥Θ”Ο”ΎΦλ―ιNi2+:‘ΎœΓΑ±Υ°÷–,ΕΓΕΰΆΣκΩ”κNi2+Ζ¥”Π…ζ≥…œ Κλ…Ϊ≥ΝΒμ,ΤδΫαΙΙ»γ”“œ¬ΆΦΥυ ΨΓΘΗΟΫαΙΙ÷–,≥ΐΙ≤ΦέΦϋΆβΜΙ¥φ‘Ύ≈δΈΜΦϋΚΆ«βΦϋ,«κ‘ΎΆΦ÷–”ΟΦΐΆΖΚΆΓΑΓ≠Γ±±μ Ψ≥ω≈δΈΜΦϋΚΆ«βΦϋΓΘ
(1)3d84s2
(2)N2ΓΓCN-(ΜρO22ΓΣΓΔC22ΓΣΓΔNO-)
(3)ΔΌΔέΔήΓΓΤΫΟφ»ΐΫ«
(4)>
(5)LaNi5ΜρNi5La
(6)ΦϊΆΦ
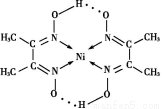
ΓΨΫβΈωΓΩ(1)ΗυΨίΙΙ‘λ‘≠άμΩ…÷Σ,ΜυΧ§Ρχ‘≠Ή”ΒΡΦέΒγΉ”(ΆβΈßΒγΉ”)≈≈≤Φ ΫΈΣ3d84s2ΓΘ
(2)ΦέΒγΉ” ΐ”κ‘≠Ή” ΐΖ÷±πΕΦœύΒ»ΒΡ «Β»ΒγΉ”Χε,“ρ¥Υ”κCOΜΞΈΣΒ»ΒγΉ”ΧεΒΡ“Μ÷÷Ζ÷Ή”ΚΆ“Μ÷÷άκΉ”ΒΡΜ·―ß ΫΖ÷±π «N2ΚΆCN-ΓΘ
(3)““œ©ΓΔ±ΫΓΔΦΉ»©ΕΦ «ΤΫΟφ–ΈΫαΙΙ,“ρ¥ΥΧΦ‘≠Ή”ΕΦ «sp2‘”Μ·ΓΘ““»≤ «÷±œΏ–ΈΫαΙΙ,Υυ“‘ΧΦ‘≠Ή” «sp‘”Μ·,“ρ¥Υ¥πΑΗ―ΓΔΌΔέΔήΓΘΦΉ»©Ζ÷Ή”÷–÷––ΡΧΦ‘≠Ή”ΟΜ”–Ι¬Ε‘ΒγΉ”,“ρ¥ΥΦΉ»© «ΤΫΟφ»ΐΫ«–ΈΫαΙΙΓΘ
(4)NiO”κFeO–Έ≥…ΒΡΨßΧεΕΦ «άκΉ”ΨßΧε,ΙΙ≥…άκΉ”ΨßΧεΒΡάκΉ”ΑκΨΕ‘Ϋ–Γ,ΒγΚ… ΐ‘ΫΕύ,άκΉ”Φϋ‘Ϋ«Ω,ΨßΗώΡή‘Ϋ¥σ,»έΒψΨΆ‘ΫΗΏ,Υυ“‘NiOΒΡ»έΒψ¥σ”ΎFeOΒΡ»έΒψΓΘ
(5)ΗυΨίΨßΑϊΒΡΫαΙΙΩ…÷Σ,ογ‘≠Ή”ΒΡΗω ΐ «8ΓΝ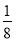 =1,Ni”–4ΗωΈΜ”ΎΟφ–Ρ,Νμ4Ηω‘Ύ…œœ¬ΝΫΗωΟφ…œ,1ΗωΈΜ”ΎΧε–Ρ,Υυ“‘Ρχ‘≠Ή”Ηω ΐ «4ΓΝ
=1,Ni”–4ΗωΈΜ”ΎΟφ–Ρ,Νμ4Ηω‘Ύ…œœ¬ΝΫΗωΟφ…œ,1ΗωΈΜ”ΎΧε–Ρ,Υυ“‘Ρχ‘≠Ή”Ηω ΐ «4ΓΝ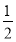 +4ΓΝ
+4ΓΝ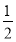 +1=5,Υυ“‘Μ·―ß Ϋ «LaNi5 ΜρNi5LaΓΘ
+1=5,Υυ“‘Μ·―ß Ϋ «LaNi5 ΜρNi5LaΓΘ
(6)ΗυΨίΫαΙΙΆΦΩ…÷Σ,ΒΣ‘≠Ή” «≈δΧε,Ρχ‘≠Ή”ΧαΙ©Ω’ΙλΒάΓΘΕχ―θ‘≠Ή””κ«β‘≠Ή”÷°ΦδΩ…“‘–Έ≥…«βΦϋ,“ρ¥Υ±μ Ψ «

 ΤΎΡ©100Ζ÷¥≥ΙΊΚΘΒμΩΦΆθœΒΝ–¥πΑΗ
ΤΎΡ©100Ζ÷¥≥ΙΊΚΘΒμΩΦΆθœΒΝ–¥πΑΗ –Γ―ßΡήΝΠ≤β ‘ΨμœΒΝ–¥πΑΗ
–Γ―ßΡήΝΠ≤β ‘ΨμœΒΝ–¥πΑΗΑ± «ΒΣ―≠ΜΖΙΐ≥Χ÷–ΒΡ÷Ί“ΣΈο÷ ,Α±ΒΡΚœ≥… «ΡΩ«ΑΤ’±ι Ι”ΟΒΡ»ΥΙΛΙΧΒΣΖΫΖ®ΓΘ
(1)ΗυΨίΆΦ1ΧαΙ©ΒΡ–≈œΔ,–¥≥ωΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ:ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ,‘ΎΆΦ1÷–«ζœΏΓΓΓΓΓΓΓΓΓΓΓΓΓΓ(ΧνΓΑaΓ±ΜρΓΑbΓ±)±μ ΨΦ”»κΧζ¥ΞΟΫΒΡΡήΝΩ±δΜ·«ζœΏΓΘ
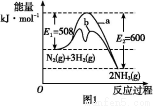
(2)‘ΎΚψ»ί»ίΤς÷–,œ¬Ν–Οη ω÷–ΡήΥΒΟς…œ ωΖ¥”Π“―¥οΤΫΚβΒΡ «ΓΓΓΓΓΓΓΓΓΘ
A.3v(H2)’ΐ=2v(NH3)Ρφ
B.ΒΞΈΜ ±ΦδΡΎ…ζ≥…n mol N2ΒΡΆ§ ±…ζ≥…2n mol NH3
C.ΜλΚœΤχΧεΒΡΟήΕ»≤Μ‘ΌΗΡ±δ
D.»ίΤςΡΎ―Ι«Ω≤ΜΥφ ±ΦδΒΡ±δΜ·Εχ±δΜ·
(3)“ΜΕ®Έ¬Ε»œ¬,œρ2 LΟή±’»ίΤς÷–≥δ»κ1 mol N2ΚΆ3 mol H2,±Θ≥÷ΧεΜΐ≤Μ±δ,0.5 minΚσ¥οΒΫΤΫΚβ,≤βΒΟ»ίΤς÷–”–0.4 mol NH3,‘ρΤΫΨυΖ¥”ΠΥΌ¬ v(N2)=ΓΓΓΓΓΓΓΓΓΓΓΓ,ΗΟΈ¬Ε»œ¬ΒΡΤΫΚβ≥Θ ΐK=ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ»τ…ΐΗΏΈ¬Ε»,K÷Β±δΜ·ΓΓΓΓΓΓΓΓ(ΧνΓΑ‘ω¥σΓ±ΓΔΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)ΓΘ
(4)ΈΣΝΥ―Α’“Κœ≥…NH3ΒΡΈ¬Ε»ΚΆ―Ι«ΩΒΡ “ΥΧθΦΰ,Ρ≥Ά§―ß…ηΦΤΝΥ»ΐΉι Β―ι,≤ΩΖ÷ Β―ιΧθΦΰ“―Ψ≠Χν‘Ύœ¬Οφ Β―ι…ηΦΤ±μ÷–ΓΘ
Β―ι±ύΚ≈ | T(Γφ) | n(N2)/n(H2) | p(MPa) |
ΔΓ | 450 | 1/3 | 1 |
ΔΔ | ΓΓ | ΓΓ | 10 |
ΔΘ | 480 | ΓΓ | 10 |
A.«κ‘Ύ…œ±μΩ’Ηώ÷–Χν»κ Θ”ύΒΡ Β―ιΧθΦΰ ΐΨίΓΘ
B.ΗυΨίΖ¥”ΠN2(g)+3H2(g) 2NH3(g)ΒΡΧΊΒψ,‘ΎΗχ≥ωΒΡΉχ±ξΆΦ2÷–,Μ≠≥ωΤδ‘Ύ1 MPaΚΆ10 MPaΧθΦΰœ¬H2ΒΡΉΣΜ·¬ ΥφΈ¬Ε»±δΜ·ΒΡ«ς Τ«ζœΏ Ψ“βΆΦ,≤Δ±ξΟςΗςΧθ«ζœΏΒΡ―Ι«ΩΓΘ
2NH3(g)ΒΡΧΊΒψ,‘ΎΗχ≥ωΒΡΉχ±ξΆΦ2÷–,Μ≠≥ωΤδ‘Ύ1 MPaΚΆ10 MPaΧθΦΰœ¬H2ΒΡΉΣΜ·¬ ΥφΈ¬Ε»±δΜ·ΒΡ«ς Τ«ζœΏ Ψ“βΆΦ,≤Δ±ξΟςΗςΧθ«ζœΏΒΡ―Ι«ΩΓΘ
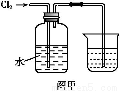
ΒγΕΤΈέΡύ÷–Κ§”–Cr(OH)3ΓΔAl2O3ΓΔZnOΓΔCuOΓΔNiOΒ»Έο÷ ,ΙΛ“Β…œΆ®ΙΐΓΑ÷–Έ¬±Κ…’ΓΣΡΤ―θΜ·Ζ®Γ±ΜΊ ’Na2Cr2O7Β»Έο÷ ΓΘ
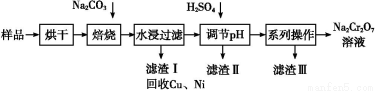
“―÷Σ:‘ΎNa2CrO4»ή“Κ÷–Κ§”–…ΌΝΩNaAlO2ΓΔNa2ZnO2Β»Έο÷
(1)Υ°ΫΰΚσΒΡ»ή“Κ≥ ΓΓΓΓΓΓΓΓ–‘(ΧνΓΑΥαΓ±ΓΔΓΑΦνΓ±ΜρΓΑ÷–Γ±)ΓΘ
(2)Άξ≥…―θΜ·±Κ…’Ιΐ≥Χ÷–…ζ≥…Na2CrO4ΒΡΜ·―ßΖΫ≥Χ ΫΓΘ
ΓΓΓΓΓΓΓΓCr(OH)3+ΓΓΓΓΓΓΓΓNa2CO3+ΓΓΓΓΓΓΓΓΓΓΓΓ ΓΓΓΓΓΓΓΓNa2CrO4+ΓΓΓΓΓΓΓΓCO2+ΓΓΓΓΓΓΓΓΓΓΓΓ
ΓΓΓΓΓΓΓΓNa2CrO4+ΓΓΓΓΓΓΓΓCO2+ΓΓΓΓΓΓΓΓΓΓΓΓ
(3)¬Υ‘ϋΔρΒΡ÷ς“Σ≥…Ζ÷”–Zn(OH)2ΓΔΓΓΓΓΓΓΓΓΓΘ
(4)ΓΑœΒΝ–≤ΌΉςΓ±÷–ΈΣ:ΦΧ–χΦ”»κH2SO4,ΓΓΓΓΓΓΓΓ,ά以ΫαΨß,Ιΐ¬ΥΓΘΦΧ–χΦ”»κH2SO4ΡΩΒΡ «ΓΓ ΓΘ
“―÷Σ:ΔΌ≥ΐ»Ξ¬Υ‘ϋIIΚσ,»ή“Κ÷–¥φ‘Ύ»γœ¬Ζ¥”Π:
2CrO42ΓΣ+2H+ Cr2O72ΓΣ+H2O
Cr2O72ΓΣ+H2O
ΔΎNa2Cr2O7ΓΔNa2CrO4‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡ»ήΫβΕ»»γœ¬±μ
Έ¬Ε» »ήΫβΕ» Μ·―ß Ϋ | 20 Γφ | 60 Γφ | 100 Γφ |
Na2SO4 | 19.5 | 45.3 | 42.5 |
Na2Cr2O7 | 183 | 269 | 415 |
Na2CrO4 | 84 | 115 | 126 |
(5)ΙΛ“Β…œΜΙΩ…“‘‘ΎΥ°ΫΰΙΐ¬ΥΚσΒΡ»ή“Κ(Na2CrO4)Φ”»κ ΝΩH2SO4,”Ο ·ΡΪΉςΒγΦΪΒγΫβ…ζ≤ζΫπ τΗθ,–¥≥ω…ζ≥…ΗθΒΡΒγΦΪΖ¥”ΠΖΫ≥Χ ΫΓΓ ΓΘ