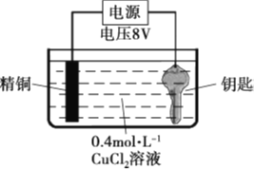
ЬтФПФкШн
ЁОЬтФПЁПCS(NH2)2 (Сђых)ЪЧвЛжжАзЩЋОЇЬхЃЌ139CЪБ ШмНтЖШЮЊ9.2gЁЄ(100gH2O)-1ЃЌПЩгУгкжЦдьвЉЮяЁЂШОСЯЕШЁЃгЩH2NCN(ЧшАБ)гыNa2SШмвКЕШзїдСЯЃЌдкдМ50ЁуCЁЂpH 10~11ЪБжЦШЁЃЌЪЕбщзАжУ(МаГжМАМгШШзАжУвбТд)ШчЯТЃК
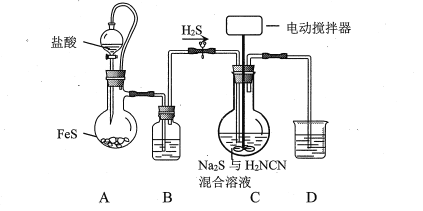
(1)зАжУBЁЂDжаЪЂЗХЕФЪдМСЗжБ№ЪЧ__________ЁЂ _________ЁЃ
(2)зАжУCКЯЪЪЕФМгШШЗНЪНЪЧ___________ЁЃ
(3)ЩеЦПжаNa2SгыЕШЮяжЪЕФСПЕФH2NCNЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_________________________________ЃЛвдКЯЪЪЕФСїЫйЭЈШыH2SЕФФПЕФЪЧ_________________________ЁЃ
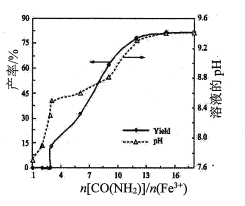
(4)ЪЕбщЙ§ГЬжаашЗжДЮМгШыH2NCNВЂМЬајЭЈШыH2SЃЌОЖрДЮжиИДжБжСвКЬхжаГіЯжаѕзДЮяЁЃЩшМЦКѓајВйзївдДгзАжУCЕФЗДгІвКжаЗжРыЕУЕНСђыхОЇЬхЕФЪЕбщЗНАИЃК______________________[ЪЕбщжаБиаыЪЙгУЕФЪдМСЃКNaOHШмвК]ЁЃ
ЁОД№АИЁПБЅКЭNaHSШмвК NaOHШмвКЃЈЛђCuSO4ШмвКЕШЃЉ ШШЫЎдЁ Na2S + H2NCN +2H2O![]() CS(NH2)2 + 2NaOH гыЩњГЩЕФNaOHЗДгІдйЩњГЩNa2SВЂЮШЖЈШмвКЕФpH ЭЃжЙЭЈH2SЦјЬхВЂЭЃжЙМгШШЃЌЯђЩеЦПжаМгШыЩйСПNaOHШмвКНСАшЦЌПЬЃЌНЋЗДгІвКзЊвЦЕНеєЗЂУѓжаеєЗЂХЈЫѕЁЂРфШДНсОЇЁЂЙ§ТЫЁЂЫЎЯДМАИЩдя
CS(NH2)2 + 2NaOH гыЩњГЩЕФNaOHЗДгІдйЩњГЩNa2SВЂЮШЖЈШмвКЕФpH ЭЃжЙЭЈH2SЦјЬхВЂЭЃжЙМгШШЃЌЯђЩеЦПжаМгШыЩйСПNaOHШмвКНСАшЦЌПЬЃЌНЋЗДгІвКзЊвЦЕНеєЗЂУѓжаеєЗЂХЈЫѕЁЂРфШДНсОЇЁЂЙ§ТЫЁЂЫЎЯДМАИЩдя
ЁОНтЮіЁП
ИљОнЬтИЩаХЯЂЃЌЗДгІашвЊдкдМ50ЁуCЁЂpH10~11ЯТНјааЃЌЗДгІЗНГЬЪНЮЊNa2S+H2NCN+2H2O![]() CS(NH2)2+2NaOHЃЌЗДгІЙ§ГЬжаЩњГЩСЫNaOHЃЌЮЊСЫБЃГжpHЕФЮШЖЈЃЌашвЊжаКЭЗДгІЩњГЩЕФNaOHЃЌдђашвЊЭЈШыH2SЦјЬхЁЃFeSКЭбЮЫсЗДгІЩњГЩH2SЃЌЩњГЩЕФH2SжаКЌгаHClдгжЪЃЌашвЊГ§ШЅЃЌDгУгкЮќЪеЖргрЕФH2SЦјЬхЁЃ
CS(NH2)2+2NaOHЃЌЗДгІЙ§ГЬжаЩњГЩСЫNaOHЃЌЮЊСЫБЃГжpHЕФЮШЖЈЃЌашвЊжаКЭЗДгІЩњГЩЕФNaOHЃЌдђашвЊЭЈШыH2SЦјЬхЁЃFeSКЭбЮЫсЗДгІЩњГЩH2SЃЌЩњГЩЕФH2SжаКЌгаHClдгжЪЃЌашвЊГ§ШЅЃЌDгУгкЮќЪеЖргрЕФH2SЦјЬхЁЃ
(1)ИљОнЗжЮіЃЌзАжУBгУгкГ§ШЅH2SЦјЬхжаЛьгаЕФHClЃЌашвЊБЅКЭNaHSШмвКЃЛзАжУDгУгкЮќЪеЖргрЕФH2SЃЌЗРжЙЮлШОПеЦјЃЌашвЊNaOHШмвКЛђепCuSO4ШмвКЃЛ
(2)ЗДгІЮТЖШдМЮЊ50ЁцЃЌзюКУЪЙгУЫЎдЁМгШШЃЛ
(3)ЕШЮяжЪЕФСПЕФNa2SКЭH2NCNЗДгІЩњГЩCS(NH2)2ЃЌИљОндзгЪиКуХфЦНЃЌЛЏбЇЗНГЬЪНЮЊNa2S+H2NCN+2H2O![]() CS(NH2)2+2NaOHЃЛЗДгІЙ§ГЬжаЩњГЩСЫNaOHЃЌЮЊСЫБЃГжpHЕФЮШЖЈЃЌашвЊжаКЭЗДгІЩњГЩЕФNaOHЃЌдђашвЊЭЈШыH2SЦјЬхЃЛ
CS(NH2)2+2NaOHЃЛЗДгІЙ§ГЬжаЩњГЩСЫNaOHЃЌЮЊСЫБЃГжpHЕФЮШЖЈЃЌашвЊжаКЭЗДгІЩњГЩЕФNaOHЃЌдђашвЊЭЈШыH2SЦјЬхЃЛ
(4)ЗДгІКѓЕУЕНСђыхЕФШмвКЃЌПЩвдЭЈЙ§еєЗЂНсОЇЕФЗНЗЈДгШмвКжаЕУЕНЦфОЇЬхЃЌПЊЪМЪБЃЌашвЊЭЃжЙЭЈШыH2SЦјЬхЃЌВЂМгШыNaOHШмвКЃЌжаКЭЕєШмгкЫЎжаЕФH2SЃЌвВЪЙОЁПЩФмЖрЕиЩњГЩСђыхЃЌЪЕбщЗНАИЮЊЃКЭЃжЙЭЈH2SЦјЬхВЂЭЃжЙМгШШЃЌЯђЩеЦПжаМгШыЩйСПNaOHШмвКНСАшЦЌПЬЃЌНЋЗДгІвКзЊвЦЕНеєЗЂУѓжаеєЗЂХЈЫѕЁЂРфШДНсОЇЁЂЙ§ТЫЁЂЫЎЯДМАИЩдяЁЃ

 КшЭМЭМЪщКЎМйзївЕМйЦкзївЕМЊСжДѓбЇГіАцЩчЯЕСаД№АИ
КшЭМЭМЪщКЎМйзївЕМйЦкзївЕМЊСжДѓбЇГіАцЩчЯЕСаД№АИЁОЬтФПЁПN2OЫзУћЮЊЁАаІЦјЁБЃЌвВЪЧвЛжжЮТЪвЦјЬхЁЃНсКЯЫљбЇжЊЪЖЃЌЛиД№ЯТСаЮЪЬтЃК
(1)аПгыМЋЯЁЯѕЫсЗДгІПЩЩњГЩN2OЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________ЃЌЦфжазїбѕЛЏМСЕФЯѕЫсгыВЮгыЗДгІЕФЯѕЫсЕФЮяжЪЕФСПжЎБШЮЊ___________ЁЃ
(2)вбжЊМИжжЮяжЪЕФЯрЖдФмСПШчЯТЃК
ЮяжЪ | N2O(g) | CO(g) | N2(g) | CO2(g) |
ЯрЖдФмСП/kJmol-1 | 475.5 | 283.0 | 393.5 | 0 |
ЂйN2O(g)КЭCO(g)ЗДгІЩњГЩN2(g)КЭCO2(g)ЕФШШЛЏбЇЗНГЬЪНЮЊ____________ЁЃ
ЂкШєЦфЫћЬѕМўВЛБфЃЌМгШыИпаЇДпЛЏМСЃЌИУЗДгІЕФьЪБфНЋ_______(ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЁЃ
(3)ЕтеєЦјФмДпЛЏN2OЕФЗжНтЃЌЗДгІРњГЬ(ВНжш)ШчЯТЃК
i.I2(g)2I(g)
ii.I(g)ЃЋN2O(g)ЃНN2(g)ЃЋIO(g)
iii.2IO(g)ЃЋ2N2O(g)ЃН2N2(g)ЃЋ2O2(g)ЃЋI2(g)
ЪЕбщБэУїЃЌдкЗДгІЙ§ГЬc(I)ЪМжеДѓгкc(IO)ЃЌгЩДЫЭЦВтЃЌЗДгІЫйТЪii_________iii(ЬюЁА>ЁБЁЂЁА<ЁБЛђЁАЃНЁБ)ЁЃ
(4)вЛЖЈЮТЖШЯТЃЌЯђКуШнУмБеШнЦїжаГфШы2 mol N2O(g)КЭ3 mol NO(g)ЃЌЗЂЩњЗДгІЃКN2O(g)ЃЋNO(g)N2(g)ЃЋNO2(g) ЁїHЁЃВтЕУN2ЬхЛ§ЗжЪ§гыЮТЖШЁЂЪБМфЕФЙиЯЕШчЭМЫљЪОЁЃ
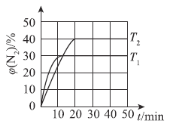
ЂйЁїH___________0(ЬюЁА>ЁБЁЂЁА<ЁБЛђЁАЃНЁБ)ЁЃ
ЂкЯТСаЧщПіБэУїИУЗДгІДяЕНЦНКтзДЬЌЕФЪЧ___________(ЬюзжФИ)ЁЃ
A.ЛьКЯЦјЬхЕФУмЖШВЛдйИФБф B.ЯрЖдЗжзгжЪСПВЛдйИФБф
C.NOКЭNO2ЕФЯћКФЫйТЪЯрЕШ D.N2OЕФЬхЛ§ЗжЪ§ВЛдйИФБф
ЂлT1ЪБЃЌИУЗДгІЕФЦНКтГЃЪ§KЃН___________ЁЃ