��Ŀ����
15��ʵ������NaOH��������250mL 1.25mol/L��NaOH��Һ����ղ���ش��������⣺
��1������ʱ����IJ��������У��ձ�����������250ml����ƿ����ͷ�ιܣ�
��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�BCAFED��
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ����
B������ƽȷ��ȡ�����NaOH����������������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
C��������ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1-2cm��
��3���������Ƶ���ҺŨ��ƫ�͵���ABC��
A������NaOHʱ���������������
B��������ƿ��ת����Һʱ��ʵ�鲽��C��������Һ����������ƿ����
C��������ˮʱ���������˿̶���
D������ʱ���ӿ̶���
E������ǰ������ƿ������������ˮ
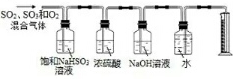
��4��ijͬѧ���ù���Na2CO3����Na2CO3��Һ�Ĺ�����ͼ��ʾ��������������Ǣ٢ޣ�
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2���������Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��3������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��4��������������ƽ��������ʱӦ�������롢����ʱӦƽ����������
��� �⣺��1�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������250mL����ƿ����ͷ�ιܣ�
�����ṩ��������֪������IJ��������У�250ml����ƿ����ͷ�ιܣ��ʴ�Ϊ��250ml����ƿ����ͷ�ιܣ�
��2�����Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�����Բ���˳����BCAFED���ʴ�Ϊ��BCAFED��
��3��A������NaOHʱ��������������̣��ᵼ��ҩƷ������ƫС����������Һ��Ũ��ƫ�ͣ�
B��������ƿ��ת����Һʱ��ʵ�鲽��C��������Һ����������ƿ���棬�ᵼ�����ʵ���ʧ����������Һ��Ũ��ƫ�ͣ�
C��������ˮʱ���������˿̶��ߣ���Һ���ƫ��Ũ��ƫ�ͣ�
D������ʱ���ӿ̶��ߣ���Һ���ƫС��Ũ��ƫ��
E��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죻
��ѡABC��
��4����������ƽ��������ʱӦ�������룬�ʢٴ�������ʱӦƽ�ӿ̶��ߣ��ʢ�����ѡ�٢ޣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

| A�� | Va��Vbʱ��c ��CH3COOH��+c ��CH3COO-����c ��K+�� | |
| B�� | Va=Vbʱ��c��CH3COOH��+c ��H+��=c��OH-�� | |
| C�� | Va��Vbʱ��c��CH3COO-����c ��K+����c��OH-����c��H+�� | |
| D�� | Va��Vb�����ʱ��c��K+��+c ��H+��=c��OH-��+c��CH3COO-�� |
| A�� | Fe3+ Na+ S2- Cl- | B�� | Al3+ NH4+ AlO2- NO3- | ||
| C�� | NH4+ Mg2+ SO42- Cl- | D�� | H+ Na+ HCO3- SiO32- |
| A�� | һ��Ũ�ȵİ�ˮ��ˮϡ�͵Ĺ����У�$\frac{c��N{H}_{4}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$�ı�ֵ��С | |
| B�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��Na2CO3��NaHCO3�����Һ�У�[c��CO32-����c��HCO3-������2c��Na+���T3c��H2CO3��+3c��HCO3-��+3c��CO32-�� | |
| C�� | ������0.4mol/LHB��Һ��0.2mol/LNaOH��Һ�������Ϻ���Һ��pH=3������Һ����Ũ�ȴ��������ϵ��c��B-����c��Na+����c��HB����c��H+����c��OH-�� | |
| D�� | ����HClO�ĵ���ƽ�ⳣ��ΪKa��̼��ĵ��볣���ֱ��Ϊ��Ka1 ��Ka2����֪��Ka1��Ka��Ka2��������Ӧ��2NaClO+CO2��������+H2O�TNa2CO3+2HClO |